Spotlight on the BIA’s influencing activity in Q1 2018
Every quarter, the BIA publishes a report – ‘Influencing and Shaping our Sector’, covering key policy developments and the BIA’s continued engagement with policymakers, regulatory authorities and wider stakeholders on behalf of the UK life sciences industry. In this blog, we take a quick look a selection of highlights from the most recent of these updates, covering the period of January to April 2018.
It’s been a busy start to 2018 for the life sciences. Brexit developments have continued apace, which we’ve been analysing every month in our Brexit briefing webinars – available on our YouTube channel. The Chancellor released his spring statement, which included the launch of two key consultations for the bioscience sector. Updated clinical trial agreement templates (mCTA and CRO-mCTA) came into effect, and NHS England held its own consultation on clinical research. Throughout all of this and more, the BIA has been continuing in our mission of enabling our members’ voices to be heard at the highest levels and building the UK into the third global cluster for life sciences. So, what were our highlights from the first quarter of 2018?
China Biotech Special Interest Group Launched
In January, the BIA launched our new China Biotech Special Interest Group, set up in collaboration with the China-Britain Business Council (CBBC) and the Department for International Trade (DIT). The group will provide a platform for BIA and CBBC members to explore opportunities for biotech with regards to Chinese collaboration and investment, with the overall objective to increase the number of UK biotech companies collaborating with, generating revenue from, and receiving investment from the Chinese market.
We had an excellent turnout at the group’s launch meeting, with people coming to hear BIA members talk about their different experiences in China. We heard from Congenica, Oxford Nanopore, Cell and Gene Therapy Catapult, Innovate UK, Kymab, and McKinsey & Company.
BIA Rare Disease Industry Group Engages Parliament
In early February, the BIA’s Rare Disease Industry Group held a roundtable in Parliament to discuss the challenges patients face in accessing innovative, potentially life-saving medicines. The group highlighted these issues to MPs and Members of the House of Lords, many of whom showed a keen interest and offered to support the group’s ongoing activities. The National Institute of Health and Care Excellence (NICE) were also represented and agreed to meet with the RDIG to discuss our concerns in more detail.
Genomics Advisory Committee Launched
At our fourth annual committee summit held in February, the BIA launched our newest advisory committee, focused on genomics. The Genomics Advisory Committee, chaired by Dr Adrian Ibrahim of the Wellcome Sanger Institute, has been established to support the strategic objective that the UK starts, scales, and builds world leading genomic businesses. It will act as a leadership platform for sharing and discussing issues of common concern between genomic businesses and will provide expert advice on important issues such as the use of data and embedding genomic medicine in the NHS.
Programme for Up-and-Coming Life Science Entrepreneurs (PULSE) Launched
In early March we launched our new three-day PULSE programme at the Francis Crick Institute. We had 25 up-and-coming life science entrepreneurs participating; 10 researchers with early stage projects selected from the Crick and 15 entrepreneurs from different stages of pre-seed investment selected from BIA referrals. The sessions were run by experienced life science professionals and seasoned entrepreneurs, largely from BIA membership.

Sector Finance Data Published
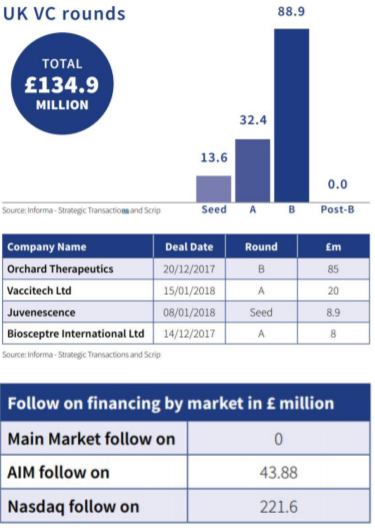
In April, the BIA released our latest quarterly Biotech Financing Update, covering the period of December 2017 – February 2018, produced with our data partner Informa Pharma Intelligence. The report shows that venture capital financing and follow on financing on the public markets are off to a solid start in 2018. It also sees the continuation of many of the trends shown in the annual report ‘Pipeline progressing: the UK’s Global Bioscience cluster in 2017’ that we released in January this year. Highlights include:
• Venture capital funding was dominated by strong B rounds that are already more than half of the 2017 total (largely made up of Orchard Therapeutics’ £85m raise), and seed funding which is already nearly half of the 2017 annual total
• Follow on financing was dominated by the GW Pharma follow on public offering on Nasdaq, which raised £221.6m
• Three UK biotech companies have used debt financing so far this year and the total accessed (£18.8m) represents around a quarter of the total from 2017
This was just a brief snapshot of our influencing activity in the first quarter. You can download the full report for a more detailed update on our work on Brexit, finance, strategic technologies, skills people and talent, intellectual property pre-clinical and clinical research, manufacturing, medicines regulation, access to medicines, and more.

.png)
.png)