CEO Update|Monday 16 March|Supporting the global effort on COVID-19
This week the BIA will host a webinar entitled ‘What can our community do to support the global effort on COVID-19?’.
It is aimed at BIA members, but others are also welcome to join me this Thursday, 11:30am. I will use this time to explain the emerging landscape as we see it.
We have already been asked by the Coalition for Epidemic Preparedness Innovations (CEPI) and the Government to co-ordinate capacity and ideas from our network in order to support the research, development, scale-up and other aspects of the life sciences sector response to the global pandemic.
Thus far the BIA has:
- Networked the UK medicines manufacturing community and convened a consortium on emerging UK capacity to engage with government, supporting the research teams from Imperial and Jenner in Oxford, to get their vaccine candidates to clinical trials scale and beyond.
- Participated in various sector-focused, government organised, coordination efforts
- Acted as an ad hoc facilitator for innovation exchange. For instance, by linking Chinese researchers inquiring about UK antibody capacity with that capacity at the request of Patrick Vallance, the Chief Scientific Officer.
- Contributed to a sector-focused conference call on the practicalities of continuing clinical trials during this period with the Department of Health and Social Care (DHSC) and the MHRA
- Briefed science and health media correspondents via the Science Media Centre on Friday on the UK ecosystem development effort which focused on manufacturing scale-up and why it takes so long at present. The briefing was attended by all major media outlets and we made ourselves a media reference point.
- Briefed Members of Parliament about what our sector is doing
- Held a call with CEPI on UK manufacturing capability – especially for innovative therapies
We are working with several parts of government on coordinating the response to COVID-19. This includes DHSC efforts on maintaining medicine supply, groups looking to coordinate patient testing capability and therapeutics via the Accelerated Access Collaborative and separate groups looking at digital therapeutics and vaccines. In addition, we are linking to global initiatives like CEPI to provide our knowhow and community linkage.
Asks of our sector as of 16 March, 2020
The NHS is looking for innovations that could help with patient testing, therapeutics, and digital therapies to reduce f2f diagnostics and vaccines, with responses by Wednesday 18 March.
They have asked us to seek information from members about relevant innovations that could help in those areas. Many of you have already shared with us your ideas or willingness to contribute. I encourage further succinct emails to me. Please be aware I plan to compile these into information I will share with the NHS and government.
Additionally, the DHSC, NHS England, and NHS Improvement are asking if there are “colleagues in academia/engineering who may have novel approaches or thinking that could help create new capacity” to manage any potential peak in demand in ventilator capacity as a result of COVID-19. Again, I am happy to pass on emails regarding this to the Government’s co-ordination team. If you can help with ventilator production contact the Business Support Helpline at 0300 456 3565 or [email protected].
Members should also be aware of a call from the European Commission for startups and SMEs with technologies and innovations that could help in treating, testing, monitoring or other aspects of the Coronavirus outbreak to apply urgently for the next round of funding from the European Innovation Council. The deadline for applications to the EIC Accelerator is 17:00 on Wednesday 18 March (Brussels local time). With a budget of €164m, this call is “bottom up”, meaning there are no predefined thematic priorities and applicants with Coronavirus relevant innovations will be evaluated in the same way as other applicants. I am assuming, this is open to UK SMEs and more information can be found here.
I hope you can join the webinar this week so I can update you on what is a fast-moving scenario. Please let me know what information you most need from us and I’ll include what I know or can find out.
Useful resources to be aware of for UK life sciences businesses
Updated government guidance
- COVID-19 Response Hub. The Government’s Response Hub includes FAQs, travel advice and guidance for health professionals. This remains the key resource for the latest information and advice. The Government is now moving to the “Delay” phase of planning. Measures include staying at home for seven days if you have a new continuous cough or high temperature.
- Guidance for the general public. The guidance issued for the general public includes a list of those ‘affected areas’ where it recommends that returning travellers stay indoors and ‘self-isolate’.
- Guidance for employers and businesses. The Government has published guidance specifically to support employers and businesses with their preparations. BEIS has launched a dedicated business support helpline for advice on minimising and dealing with the impacts of coronavirus. They can be accessed at 0300 456 3565 Monday to Friday, 9am to 6pm, or email [email protected].
- NEW: The MHRA have published a blog post, “Advice for Management of Clinical trials in relation to Coronavirus” MHRA is aware that there are challenges arising in relation to Coronavirus and the effect this is having on the conduct of clinical trials. They recognise the difficulties this creates for managing trials and would like to offer some advice.
- NEW: The Health Research Authority has produced new guidance for sponsors, sites and researchers about the COVID-19 pandemic. The guidance covers the setup of new studies, amendments to existing studies and changes being made by sponsors at this time. The guidance will be updated regularly.
- NEW: The DHSC has stood up the National Supply Disruption Response (NSDR) to monitor the supply situation and co-ordinate actions to address any incidents of supply disruption where normal procedures are unable to provide a resolution. Marketing Authorisation Holders will have received guidance directly from the Department today. They NSDR can be contacted at: 0191 283 6543 and at [email protected].
- NEW: The National Credentialing Register has published a list of statements from Hospital Trusts on policies restricting ‘external’ representatives visiting their sites. This includes the contacts at the trusts that enquiries need to be sent to.
- NEW: DHSC issues guidance on the impact on COVID-19 on research funded or supported by NIHR. This will mean that many research studies funded by NIHR, or supported by NIHR (via the Clinical Research Network and other NIHR infrastructure) may need to be paused, to free up NIHR-funded staff to help bolster the frontline response to COVID-19. In addition, we recognise that NIHR award-holding organisations may need to pause research studies for locally determined public health reasons. However, clinical trials or other research studies which are funded or supported by NIHR should continue if discontinuing them will have significant detrimental effects on the ongoing care of individual participants involved in those studies. These are clinical decisions which will need to be made on a case-by-case basis by local decision makers on the basis of local risk and capacity assessments.
Guidance on the legal aspects of COVID-19 by Simmons & Simmons
- COVID-19: risks and opportunities
- Remote working - signing legal documents using e-signatures
- Government websites for country-specific COVID-19 guidance
- The Coronavirus outbreak challenge
- Coronavirus – impact on business contracts
- Construction and the Coronavirus
- Trusting the internet: An overview of anti-disinformation laws
- Coronavirus (COVID-19) - the insurance issues
- Managing Insolvency Risk and Financial Reporting
- Coronavirus - impact on tax
- COVID-19 and its potential impact on trade finance
- Other publications
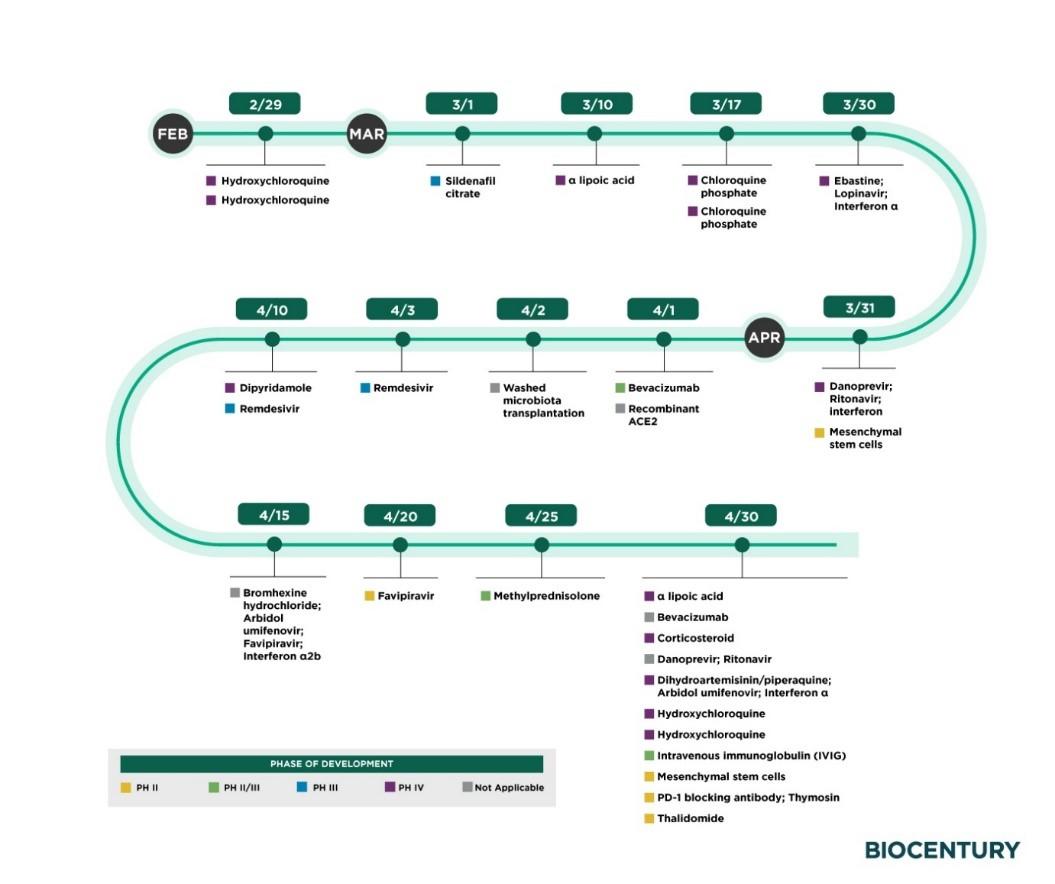
A resource I’ve found useful, is by the trade magazine BIOCENTURY which has developed a free-to-access portal, which is especially strong on global development on therapeutics and vaccines for COVID-19. The graphic below sets out expected global trial timelines for repurposed drugs which I’ve found particularly helpful.
The BIA as an organisation is moving to a more virtual way of operating as a result of the pandemic and we have cancelled or postponed face-to-face events in the coming weeks. Our office is currently closed to visitors and members, however we remain committed to ensuring our ecosystem can influence and connect at this important and unusual time, and are working on novel ways to do that. This week’s webinar is our first effort at that and further ideas from members are welcome.
Looking forward to connecting soon.
Best
Steve
.png)
.png)
