Life Sciences Industrial Strategy & Sector Deal Newsletter | October 2018

Welcome to the October edition of the Life Sciences Industrial Strategy & Sector Deal Newsletter. Here you will find an update on how we are progressing with the implementation of phase 1 of the Life Sciences Sector Deal as well as our upcoming priorities and engagement opportunities.
Upcoming Priorities
Government is developing an update to the Life Sciences Sector Deal, and we anticipate announcing the next phase of Life Sciences Industrial Strategy implementation as part of this. We are engaging with the sector on the development of proposals through:
- Expert groups that report to the Life Sciences Council (see below for further details on the expert groups)
- The trade associations
- Professor Sir John Bell, as Life Sciences Champion
Key themes being considered for the update by the Life Sciences Industrial Strategy Implementation Board and the Life Sciences Council include: Early Diagnostics, Clinical Trials, Innovative Regulation, Skills, Growing UK Clusters, and AI in Health.
Delivery of the Life Sciences Industrial Strategy and Sector Deal: Progress Highlights
The Industrial Strategy Challenge Fund (ISCF) is aligning with the Life Sciences Industrial Strategy to deliver shared objectives. With funding channelled through United Kingdom Research and Innovation, the ISCF is a core pillar of the government’s modern industrial strategy, committed to increase funding in research and development by £4.7 billion over 4 years to strengthen UK science and business.
Digital Innovation Hubs:
- Health Data Research UK’s role in delivering the Digital Innovation Hubs (DIHs) formally began on 1st September, following their recent announcement of a £37.5m investment in DIHs through the ISCF.
- Health Data Research UK (HDR UK) is seeking input from key stakeholders and organisations to define the specification for the architecture, data and technical standards, and governance frameworks of the DIHs. As part of this dialogue phase, an academic design workshop was hosted on 5th September and a technology sector symposium took place on 2nd October 2018. Future events and activities will be organised to support this dialogue, including a webinar on the DIH programme which will run on 15th November. Register for the webinar here.
- HDR UK have launched a competition for the Digital Innovation Hub Sprint Exemplar Innovation Projects as part of phase 2 of the DIH programme. These projects aim to identify and develop the processes, technical solutions, knowledge and skills needed to deliver robust, secure and scalable solutions to inform delivery of the Digital Innovation Hub Programme. HDR UK aim to support the delivery of 6 to 8 projects in total, with available funding of around £100,000 to £400,000 for each successful project. Further details can be found on the MRC website.
AI and Data Grand Challenge Mission:
- The first iteration of the Code of Conduct for AI and data-driven healthcare technology was published on 5th September; the full guidance can be viewed here.
- The code aims to clarify what we expect from our technology partners, in terms of commercial arrangements and standards, to ensure we enable technology to deliver the best possible outcomes for patients and the system. It is a key step in Government’s commitment to delivering on the Prime Minister’s mission to use data, artificial intelligence and innovation to transform the prevention, early diagnosis and treatment of chronic diseases by 2030. The Code aims to:
- Provide clarification on what we expect from suppliers of data-driven technologies, and what the government will do to support and encourage innovators in health and care, including the development of trusted approval systems and a coherent pathway for suppliers to enter the market.
- Provide the basis for ongoing engagement and conversation on how we should use new technology to provide better and more sustainable services, with our partners in academia, industry and the health and care system, patients, clinicians, and the wider public.
- Provide the basis for the health and care system and suppliers of digital technology to enter into commercial terms in which the benefits of the partnerships between technology companies and health and care providers are shared fairly.
Government has launched a questionnaire alongside the Code of Conduct to enable stakeholders to provide real-time feedback. The Code will be amended and evolved over the coming months in line with this feedback, with the next iteration expected in December.
Cell & Gene Therapy Catapult:
- Government has announced a £780m investment into the UK’s catapult network, which will include a £70.6m investment in the Cell and Gene Therapy Catapult over the next 5 years. This builds on the £12m investment awarded from the Industrial Strategy Challenge Fund (ISCF) for the expansion of the Catapult’s manufacturing centre in Stevenage, doubling the centre’s capacity – part of Government’s commitment in the Life Sciences Sector Deal to support the growth of medicines manufacturing, for which a total of £146m has been allocated from the ISCF.
- The Stevenage Cell and Gene Therapy Catapult manufacturing centre has been given the green light to produce new cell and gene therapies by the Medicines and Healthcare products Regulatory Agency (MHRA). The licences will enable the development of cell and gene therapies and support faster progression of therapies to clinical trials and patients.
Accelerated Access Review:
- Two ISCF competitions have been announced as part of the Digital Health Technology Catalyst (DHTC), which committed £35m of ISCF funding over 4 years. The DHTC is a core element of Government’s plans to implement the Accelerated Access Review.
- Digital health technology catalyst round 3: feasibility studies. Up to £1m of ISCF funding is available to support feasibility studies that can potentially develop new digital technology solutions to healthcare challenges. The competition opened on 3rd September and will close on 31st October 2018: find out more here.
- Digital health technology catalyst round 3: collaborative R&D. Up to £8m of ISCF funding is available to support industrial research and experimental development projects that develop new digital technology solutions to healthcare challenges. The competition opened on 3rd September and will close on 31st October. Click here for more details on how to apply.
ISCF Competition Round Up
- Digitalisation of medicines manufacturing. Up to £8 million has been made available from the Industrial Strategy Challenge Fund to support the growth of the UK’s commercial capacity to manufacture medicinal products through the application of digital technology. The competition opened on 10th September and closed on 10th October. See here for further details.
- Digital Pathology Centres (ISCF Wave 2): The competition for creating a network of digital pathology, imaging and AI centres is now closed. Shortlisted consortia were invited for interview on the 17th and 18th September 2018.
- ISCF Wave 3 update: Work is ongoing to refine and adapt the initial proposals, which will form the final list of compelling challenges. Some potential challenges, although not all, held open engagement events to refine the process. The final shortlist will be announced in due course.
Updated Governance Framework
The Life Sciences Industrial Strategy Implementation Board (LSISIB), chaired by Lord Henley and Prof Sir John Bell, successfully met again on 4th September to discuss progress on Government’s Sector Deal commitments. The meeting included a presentation on next steps on Digital Innovation Hubs, led by HDR UK’s Director, Andrew Morris. The next meeting is due to take place on 29th October.
The Life Sciences Council (LSC), chaired by Greg Clark and Matt Hancock, is due to meet again on 21st November 2018. The Expert Groups have been offered the opportunity to propose substantive agenda items for discussion at the Life Sciences Council. Our updated governance framework is as follows:
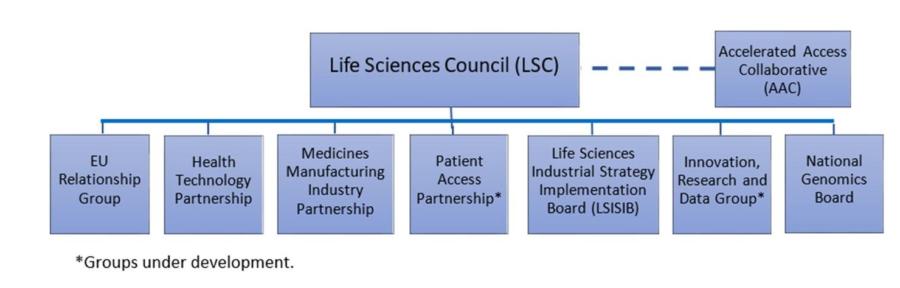
The Health Technology Partnership, chaired by Lord O’Shaughnessy and Michelle Brennan, J&J, will meet on 9th October.
Other News from OLS & the Sector
- Wellcome is to launch a new £250m, not-for-profit fund that will place big bets on ambitious research programmes with the potential to fundamentally change science or transform health over a five- to ten-year horizon. The Wellcome Leap Fund will support researchers from around the world to pursue bold ideas at scale and speed, drawing inspiration from the technology and venture capital industries.
- Greg Clark, Secretary of State for Business, Energy and Industrial Strategy, and Lord O’Shaughnessy, Parliamentary Under-Secretary of State for Health, attended the opening of the Novo Nordisk Research Centre in Oxford, a new centre for research which aims to develop a new generation of medicines that will transform the lives of people living with diabetes. Novo Nordisk will invest £115m over 10 years, building on Government’s commitment in the Life Sciences Sector Deal to work with industry to increase spending on R&D investment in the UK. Read more about the Centre here, which was officially opened on 12th September.
- As part of the Ageing Society Grand Challenge (ASGC), the ASCG team are looking to gather industry views on the strategies they are developing to respond to demographic change. The team would be keen to hear from companies on the following:
- How your business is seeking to respond to the wider opportunities and challenges of ageing, including issues such as supporting an older workforce, and
- How you plan to capitalise on new growing domestic and international markets emerging from people living longer across the world?
If you are interested in getting in touch, please send responses to Mark Nassar and Charlotte Bright at [email protected].
.png)
.png)