Tech Bio

This explainer booklet gives an introduction to the exciting BIA member companies that are working at the interface of biotechnology and technology, and shows how they are already making an impact on drug discovery and patient care. Data-driven life sciences technology or tech bio is an area where the BIA is rapidly building its expertise and we will be working hard to bring together all elements of the biotechnology landscape to ensure that these companies have the right policy framework and the investment that they need to grow and succeed. We have interviewed our members to inform the content of this booklet and would like to thank them for their insights and for sharing just some of the fascinating work they doing to bring tech bio to life.
Please download the full Tech Bio report for some case studies of incredible companies working at the interface of biotechnology and technology.
Get in touch with Michael McGivern, Head of Membership [email protected] for more information about getting involved.

Foreword from NHS England Chairman, Lord David Prior

The COVID-19 pandemic has had an unprecedented impact on healthcare systems around the world. The rapid and effective response from the National Health Service (NHS) and our life sciences industry working together has led to the UK being at the forefront of finding solutions to tackle this terrible pandemic. The use of data has been critical to our understanding of the virus and its impact, and this data has underpinned the tremendous efforts of academics and industry to develop COVID-19 vaccines.
Transforming the future of healthcare will rest on the new technologies and therapies emerging within the life sciences sector. Developing the innovations we are seeing in data-driven life sciences technology will be pivotal as we transform drug discovery pathways and patient care. If we are to succeed with this transformation, then it is critical that we bring patients with us on the journey. By ensuring transparency and through continued engagement, we will build patient confidence in sharing their data in order to ultimately drive better health outcomes for all.
The use of data-driven life sciences technologies is central to the UK government’s Life Sciences Vision. We foresee an NHS where research is embedded as a core part of effective patient care to deliver our goal of a digitally enabled and pro-innovation clinical research environment. To ensure that the NHS can flourish as an innovation partner, we will be looking carefully at how the NHS enables access to patient data to aid research and that we share data in a format that is research-ready.
This report shows that the NHS gives the UK a unique opportunity, and a key global advantage, to facilitate patient-centred research and development. Collaboration between NHS and life sciences companies is already changing health outcomes and there is a huge potential to transform future healthcare for patients here in the UK and around the world. With controlled access to NHS data, we can support data-driven life sciences companies to create a more cost-effective and efficient healthcare system as we move into the future.

Defining and understanding

The aim of this report is to introduce and explain the impact that data-driven life sciences companies are having on drug discovery and patient care. Data-driven life sciences companies work at the interface between biotechnology and technology – so another way to describe companies is to take the ‘tech’ of technology and the ‘bio’ of biotechnology to create ‘tech bio.’ They combine cutting-edge techniques from both sectors to draw insights from a wealth of data, including data concerning patients, drug molecules, healthcare infrastructure and research and development, to inform and transform drug discovery and patient care
These companies are producing data-driven technology that will enable the healthcare system to deliver more personalised healthcare to more patients, more quickly, and at a lower cost.
These companies could also be labelled as digital health or data technology companies but neither of these terms quite captures both the importance of data that underpins them and the impact they will have on improving health outcomes.
The work of these companies and their ground-breaking technologies has never been more relevant. The COVID-19 pandemic increased interest in how health data can be collected and the benefits of using this data to rapidly develop vaccines, manage healthcare resources, and repurpose existing drugs.
The UK government is now working to capitalise on the innovation inspired by the pandemic response, using it as a blueprint for the UK Life Sciences Vision launched in July 2021. This sets a 10-year strategy for the life sciences sector to accelerate delivery of innovations to patients, much of which will come from data-driven life sciences companies.
People are seeing that donating and sharing their data can play a direct role in improving health outcomes, similar to donating blood. Despite game-changing advancements in data-driven life sciences during the pandemic, the public still have concerns over how their data is managed and shared. Building trust and maintaining transparency continue to be vital to reassure people that their data is not being exploited for negative purposes and persuade them to continue sharing it.

Opportunities and obstacles
BIA Member companies give many reasons for choosing to be based in the UK. These include:
- opportunities for NHS collaboration in research and development
- government funding, including Innovate UK
- the breadth of excellent science coming out of UK academic institutions
- access to science and technology talent
- well-developed infrastructure
- ties to Europe
- growing public and private investor base
- the MHRA as a highly respected regulator
- well-developed scientific clusters that attract international talent and innovation.
Despite these benefits, UK-based data-driven life sciences companies face the challenge of a fragmented healthcare system, as well as complexities in overall responsibility for managing data and technology within healthcare. The NHS, which is rich in health data, places the UK in a unique global position. However, the NHS has faced issues in how to collect, curate and ultimately share this data to make a positive impact on patient health. Many bodies exist in and around the NHS, but with no centralised hub or controlling body, it is difficult for companies to know where to target their resources to access the data.
Addressing the barriers to success
The UK government is taking steps to address these challenges. Its Life Sciences Vision sets out preconditions to address the fragmentation of the healthcare system and boost support for data-driven life sciences companies.
Several examples already show these barriers can be overcome and that collaborations between industry and the NHS have the potential to transform the future of patient care. The RECOVERY Trial and the development of Test, Track and Trace have demonstrated the value of using healthcare data to fight the COVID-19 pandemic. The NHS has also worked with Palantir to better manage NHS resources during the pandemic. Data-driven healthcare is already making a difference outside the pandemic response. The government is collaborating with pharmaceutical company Novartis to tackle heart disease – a leading cause of death in the UK.

Case studies
Showcasing data-driven life sciences successes
The following six BIA member case studies illustrate just some of the innovation coming out of UK data-driven life sciences companies:
-
Jiva AI has created a multi-modal data platform that allows scientists and clinicians to integrate new streams of data into their research.

Jiva AI is headquartered in Cardiff since 2019. CEO: Dr Manish Patel
What does the company do?
Jiva AI describes its platform as a low code/no code platform with interfaces that clinicians with no coding or tech knowledge can easily use.
Manish told us: "Jiva AI is like the Microsoft Office of AI, giving people the tools and ability to create their own AI models, and then deploy these themselves – we hope this platform will help to democratise AI."
How does the technology work?
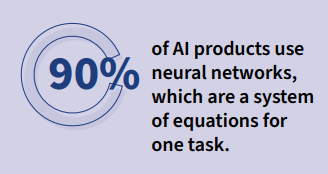
90% of AI products use neural networks, which are a system of equations for one task.
You can’t get them to recognise something new – you have to teach them. Jiva is the world’s first multimodal AI platform which can integrate/unify different AI models. This means a doctor with imaging data can integrate models from other data verticals (for example genetic data) into their existing AI to build a fuller picture of the patients being treated.
How has the technology been used?
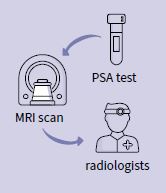
JivaRDX uses AI to identify and stratify prostate cancer cases using MRI imaging.
In the UK, and much of the world, the prostate cancer clinical pathway involves a PSA test and, if you’re beyond a certain PSA level, you go for an MRI scan. Then a set of radiologists look at your scan and decide whether you need a biopsy. This radiologist stage is very subjective and can lead to unnecessary biopsies. 80% of patients who have a biopsy get some kind of complication, which could include erectile dysfunction, rectal bleeding or even sepsis.
Manish said: "This is where AI can aid clinicians in their decision making and reduce the rate of unnecessary biopsies by drawing on data captured at the radiology stage. JivaRDX can outline regions of tissue that are predicted to be cancerous in prostate MRI scans. JivaRDX automatically annotates imaging files and requires minimal intervention and training, so it can easily be integrated into current radiology practice. It is currently going through clinical trials and Jiva plans to add in the modalities of ethnicity and PSA levels to further enhance the decision-making support available to clinicians."
The future
Jiva plans to split out the prostate cancer side of the business to allow it to focus on building the platform, which they see as a game-changer in the healthcare data technology space.
Manish added: "We want to see other people creating models like we have in prostate cancer using this platform."
- DeepMatter’s ICSYNTH software supports scientists in the lab to automate their chemistry and supply them with real-time information on their experiments, allowing for better discovery insights.

DeepMatter is headquartered in Glasgow since 2015. CEO: Mark Warne
What does the company do?
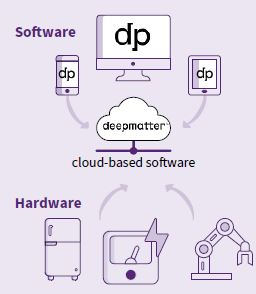
DeepMatter builds software called ICSYNTH and an integrated cloud-based software and hardware ‘internet of lab things’ platform called Digital Glassware® to improve the productivity of scientists working in drug discovery.
Mark explained: "Our products collect, structure and analyse data to be able to control robotics to automatically carry out manual repetitive nonvalue-creating tasks. This allows scientists to do something more value-creating and enables them to focus on discovery insights."
How does the technology work?
DeepMatter has created cloudbased software to collect data from the chemist. The company also designs hardware that chemists use in experiments to capture data such as temperature, pressure and stir rate as well as a a range of unique data feeds in real time.
Mark explained: "The lab hardware acts like a sort of Fitbit for chemistry, allowing access to streams of data you didn’t have before. This data is streamed up to the cloud and then essentially handed back to the chemists in easily usable formats to help inform them to make decisions."
How has the technology been used?
DeepMatter’s software and hardware are already supporting companies to manage their drug discovery processes in new ways and improve existing processes.
Senior scientists at the Flexible Discovery Unit and Chemical Development, GlaxoSmithKline GSK, Stevenage, said: "ICSYNTH supports us as an integral part of our synthesis route discovery process and already helped us solving tricky organic synthesis challenges. Moreover, it provides us with many – sometimes unconventional – ideas that we would not have come up with ourselves."
Paul Wood, Tocris Biotechne Lab, said: "Access to real-time data is important in the lab because it lets you see what’s happening with your chemistry so you can take corrective action straight away if necessary. The photo notes are useful for taking pictures of experimental setups and for taking pictures of colour changes in the reaction as things progress. Gathering unsuccessful reaction data is also important because then we can then go back and look through the data and try to take corrective action to be able to reproduce the reaction in the future."
The future
The company sees their technology supporting a move to far more autonomous chemistry in the future.
Mark said: "As we build a data repository, we learn more about the interplay between the chemist and the chemistry – and can move towards essentially more autonomous chemistry. However, it’s always important to point out that chemistry is fundamentally a dangerous and highly skilled business, so the chemist still plays a central role in steering the chemistry in the lab"
- Precision Life uses population-scale data to drill down into how diseases work to better support clinicians in identifying drugs and treatments for individual patients.

Precision Life is headquartered in Oxford since 2014; CEO: Steve Gardner
What does the company do?
Precision Life describes its platform as working ‘from populations to personal’.
Steve told us: "We use population-scale data to understand what is driving disease within patient subgroups. We then use that information to make better-informed decisions about what is going to be an effective intervention for an individual patient."
How does the technology work?
We take very large-scale patient populations and stratify them by genomic, epidemiological and other types of data to understand what’s driving disease for each of those groups. We’re then able to come up with an understanding of what’s going to be the most effective precision medicine strategy for that group. You then have to put that knowledge in the hands of either a clinician or a patient to inform decision making at this level.
How has the technology been used?

Type 2 diabetes is a $1.75 trillion problem, accounting for 15% of US and 10% of UK healthcare spending. But 80% of that is spent treating complications of the disease, including cardiovascular disease, Alzheimer’s and dementia, renal failure or blindness and glaucoma. To date, nobody has been able to predict or to find the genes that are associated with those complications.
Steve said: "We did a study at the UK Biobank of 2,900 patients with type 2 diabetes who had severe complications and 5,800 who did not have those complications. We found 35 mutations in 20 genes, 10 of which are druggable. We were also able to build a prediction model that at the point of diagnosis, or even when somebody goes into prediabetes, could be used to predict whether they’re going to get complications in the future."
Sharing these insights with doctors cuts costs as they already know the interventions that are effective, whether they are existing drugs or monitoring. Monitoring glucose three times a day or with a continuous glucose monitor reduces all these complications by 14%. Blood pressure checks reduce renal failure by 80% and annual eye check-ups reduce glaucoma and the development of blindness by 90%.
Steve added: "These are smart ways of using this insight that you’ve generated at an individual patient level and can also be used to empower patients to monitor their own health."
The future
Precision Life is focused on three key audiences with its technology: biotech and
pharma, clinical development and diagnostics, and healthcare.
Steve added: "There is a biology revolution coming and there will be a fundamental shift in the level of detail at which you can understand complex chronic diseases that cover 80-85% of spending in healthcare."
- Synthace support biologists in the lab to automate their experiments and supplies them with real-time information to change the way science is done and communicated.

Synthace is headquartered in London since 2011. CEO Guy Levy-Yurista and CSO Markus Gershater
What does the company do?
Life scientists are working with the most complex phenomena and do so primarily using handheld tools and Excel. Synthace wants to change this using 21st century digital and automation tools.
Markus explained: "Simply, it’s about doing life science the way it should be done."
Guy added: "Our company is about removing barriers to innovation that research and development (R&D) life scientists currently face. We help life scientists address complexity, speed and reproducibility in their overall R&D lifecycle."
How does the technology work?
digital protocols that simplify the process of recreating an experiment. When scientists log on to the Synthace Life Sciences R&D Cloud, they can program their experiment in an intuitive graphical user interface by building together blocks of functionality. This produces a welldefined methodology to send to the lab so that the experiment can be carried out anywhere in the world using automation. Synthace then automatically gathers the experiment results, along with other useful information on exactly what happened during the experiment.
Markus explained the benefits: "We can contextualise the data that are produced, making it so much more robust and richer. This data is the foundation on which R&D teams can base their transformational insights."
Being cloud-based, the technology means experiments can easily be reproduced and adapted by other scientists.
How has the technology been used?
Oxford Biomedica selected Synthace to improve the way it produces viral vector (a biological tool used to deliver genetic materials into cells). By using the Synthace Life Sciences R&D Cloud, Oxford Biomedica has increased the amount of product it can produce with each experiment by 3-10 times, reduced variability in the viral vector produced by 5.5 times, and reduced the resources used to produce the vector by 32%.
André Raposo, DPhil Computer-Aided Biology Group Lead, Oxford BioMedica, said: "Synthace allows us to think outside the box and do things we couldn’t do before."
The future
Guy said: "The interesting thing about this notion of a cloud is it’s not just a deployment form factor. It a computation environment that allows you to share information and so changes the way science is done and communicated. We see Synthace becoming this giant that really helps progress life sciences."
- Healx uses its platform of carefully curated rare disease and drug data to identify drugs that could be repurposed for treating rare diseases.

Healx is headquartered in Cambridge since 2014; Co-founder: David Brown
What does the company do?
More than 350 million patients worldwide live with one of over 7000 unsolved rare diseases, and 95% of these patients lack a treatment option for their condition.
David said: "What we’re trying to do at Healx is improve the efficiency, enhance the scale and reduce the cost of matching drugs to diseases by at least tenfold. Of course, the methods we develop to do that can then be applied to more common diseases as well, but our focus is on rare diseases, where there is a huge unmet need."
How does the technology work?
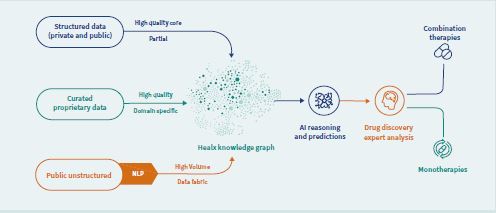
Healx’s Healnet platform uses several cutting-edge AI algorithms that are based on analyses of how diseases function in the body and the world’s most comprehensive database for rare diseases. The Healnet system also houses data on how a wide range of existing compounds work on cells in the human body. The AI algorithms use all this information to match known compounds to diseases. Importantly, the platform acts in a hypothesis-free and human bias-free way and so it can identify targets and drugs that would not usually be considered for the disease under investigation. Redeveloping approved drugs, for which safety and handling in the body is already known, gets drugs to patients faster and more costeffectively than discovering new drugs. The use of AI also means the team can run more research programmes at scale.
How has the technology been used?
Healx has worked with several rare disease patient groups, including the USA-based non-profit FRAXA, to identify drugs to treat Fragile X, the most common inherited cause of intellectual impairment and the most common monogenic cause of autism.
Dr Mike Tranfaglia, Chief Scientific Officer and Co-Founder, FRAXA Research Foundation: "The Healx partnership has been nothing short of amazing. In less than two years together, we were able to deliver decadesworth of drug discovery, and now we’re taking those discoveries to the clinic."
Not only has the Healx technology identified potential drugs to treat the condition but the sophisticated algorithms are also able to identify combinations of drugs which, when used together, could be used to treat Fragile X more effectively. Healx will be taking the results of its research into clinical trials later this year.
The future
Healx believes its tech-driven approach is at the forefront of a new generation of drug discovery.
David explained: "Supervised machine learning will start to transform drug discovery. Machines will be fed information that humans are discovering, the machine will recognise the patterns in diseases and automatically match the molecules to treat them."
- Lifebit works with biobanks around the world to enable their data to be accessed by scientists in a safe and secure way

Lifebit is headquartered in London since 2017. Vice President, Commercial: Thorben Seeger
What does the company do?
Lifebit is working with biobanks around the world to enable their data to be accessed by scientists.
Thorben explained: "Lifebit is working with biobanks around the world to enable their data to be accessed by scientists. We make sensitive clinical genomic data available in a secure and usable way to allow researchers to develop drugs and better treatments. Scientists can run any type of analytics over the data, so basically it’s as if they had it in their hands but they can’t take the actual data out, they can only take the insights out."
How does the technology work?
Traditionally, data was stored in large, siloed datasets in lots of different places. Data often went unused due to the challenges in accessing it. To use the data, it had to be moved and centralised in one place to allow scientists to run analytics. As genomics and precision medicine have taken off, we have seen datasets explode in size, so centralisation is no longer feasible or practical. This is where Lifebit comes in as it enables computational analysis of the data where it resides without having to move it from its secure environment.
How has the technology been used?
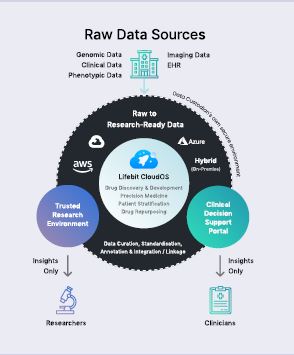
In the UK, during the COVID-19 pandemic Lifebit has worked with the UK government and Genomics England to deliver access to its datasets for eight leading pharmaceutical companies – as well as research organizations – to fuel vaccine, treatment and early-detection research. Genomics England needed a federated data analytics system to make that data available to multiple parties. Today, pharmaceutical companies and researchers can access the cohort and connect their own private datasets to research as if that data is in one place.
Lifebit is also working with Hong Kong’s Genome Institute (HKGI) to support its implementation of the Hong Kong Genome Project. The Lifebit platform will create various portals that cater to different end-user needs:
- Clinical Portal will provide clinicians with critical clinical decision support, enabling HKGI and its clinicians to turn raw data into key patient insights.
- Research Portal will enable biomedical researchers worldwide to securely access, query and analyse the extensive dataset to increase the understanding of diseases including undiagnosed disorders and hereditary cancers.
The future
The company will continue to focus on international expansion.
Thorben added: "In the very near future, we will be powering the majority of these programmes around the world and become the key gateway to and quality enabler of data-driven research."

Improving the UK landscape
The UK government has recognised the opportunity of data-driven life sciences for the UK with the Life Sciences Vision, which highlights the need “to harness the UK’s unique health data”, seeking to simplify the oversight of NHS health data to drive research and innovation.
The Life Sciences Vision has been followed by the first national artificial intelligence (AI) strategy in the UK. The 10-year plan seeks to strengthen the country’s position as a global leader in AI innovation, regulation and adoption. Also, the UK’s National Data Strategy, the Data Saves Lives policy paper, and the upcoming Goldacre Review will further promote and embed broader use of health data for research and innovation. Together, this activity represents a clear drive to make the UK a global leader in AI and data-driven life science.
To support this, the BIA has specific policy ‘asks’ that would better enable data-driven life sciences companies in the UK to grow and scale. The UK is at the cutting edge of data-driven life sciences but competition for global investment is strong.
- The UK government should ensure the UK is fertile ground for private investment here by expanding R&D tax credits to include data and cloud computing costs and support early-stage projects with innovation grant funding, which leverages downstream private investment.
- There is a rich ecosystem of data-driven life sciences companies in the UK with the potential to become the next generation of global giants. Government agencies and NHS bodies should have a unified strategy that champions UK data-driven health SMEs and supports their growth by providing access to projects, contracts and data.
- Countries that wish to be at the cutting edge of innovation and see benefit for society must embrace new technology and approaches. Regulators should be agile and responsive to the needs of innovative data-driven life sciences companies, streamlining and facilitating the route from innovation to market.

Transforming health ecosystem
This report has explored how data-driven life sciences companies are using technology to lead transformation from early-stage research and development, through supporting clinicians to diagnose diseases, to repurposing existing drugs to address unmet medical need.
But how could technology transform the broader healthcare ecosystem in the future?
This question is addressed by the Deloitte Centre for Health Solutions research report ‘Predicting the future of healthcare and life sciences in 2025: The future unmasked’. A common thread throughout the ten predictions in the report is the huge acceleration in pace and scale of technology-enabled transformation. Here, we highlight some of the predictions that are underpinned by technology.
-
Companies reverse the decline in the returns from pharma R&D: Future pharma R&D processes will use AI-enabled digital platforms, while skills and talent will be enhanced through research partnerships with academia, AI for drug discovery companies and digital tech companies.
- Clusters of trusted partnerships accelerate innovation: Clusters that bring together key players in industry, academia and health provision will accelerate the pace of digital transformation. Trust, efficiencies, increased access and reduced costs will be driven by new standards for data sharing, analysis and transparency.
- MedTech and the Internet of Medical Things are crucial drivers of value-based care: MedTech companies will drive the future of health, developing transformative technology that enhances products/services and enables 4P medicine.

.png)
.png)
