TechBio 2.0
Showcasing the next generation of data-driven life science companies that use cutting-edge technologies to transform drug discovery and patient care.
TechBio companies combine cutting-edge techniques from both sectors to draw insights from a wealth of data, including data concerning patients, drug molecules, healthcare infrastructure and research and development. This data is not only being used to inform and transform drug discovery and patient care but, as we will see in this update, TechBio companies are also addressing challenges faced by industry and agriculture, such as creating cleaner and greener chemicals and materials.
Since our first report, “TechBio: How data-driven life sciences companies are transforming drug discovery and patient care”, activity in this part of the sector has continued at pace. Public and private investors are already recognising the potential that TechBio companies have to drive forward the UK’s bioeconomy. In the last year, flurry of new companies has secured investment and been established, some of whom are profiled in the new TechBio 2.0 report.
Read the Tech Bio 2.0 report for developments in data sharing, inspiring case studies and the future of techbio!
Foreword

Mike Snowden, SVP Discovery Sciences, BioPharmaceuticals R&D, AstraZeneca
Data has always been at the core of drug discovery and development. Since the days of ‘analogue’ drug discovery we have used techniques like organ baths, IC50s and myography to observe physiology and create a structured way of finding and prioritising medicines that will most effectively treat patients.
As technology became more sophisticated the industry increasingly focused its attention on understanding the core building blocks of biology: DNA, RNA and proteins, generating omic signatures and building network maps and knowledge graphs that allow us to interrogate the true underlying mechanisms of disease and medicines.
The increasing adoption and application of Machine Learning and Artificial Intelligence (AI) to these data has started to address this issue, but raises new questions about the introduction of bias and the increasing ‘black-boxification’ of life sciences.

Extrapolating to the future we can speculate that as data generation continues to outstrip our ability to process and interpret data manually, we must increasingly rely on unbiased data interpretation to guide how we develop medicines and technology solutions to address big patient challenges.
Here a mindset shift towards the acceptance of the ‘digitization of biology’, will be facilitated by the coming-together of the TechBio ecosystem. In this ecosystem players will contribute and learn how we can collectively accelerate access to a new generation of tailored medicines and solutions that draw on the rich breadth and depth of the data now available to us.
At AstraZeneca we welcome the update to the BIA’s TechBio report, which showcases new demonstrations of how the convergence of AI, data and biology (both organic and synthetic) can deliver a new wave of innovative science. Establishing a thriving ecosystem of established industry players, academics, startups and technology companies allows us to collectively build the appropriate frameworks to develop safe and efficacious treatments, and supports the UK’s ambition to become a world-leading Science Superpower, as set out in the Government’s Life Sciences Vision. Seeing this ecosystem flourish in the UK will continue to be dependent on building the sector skill-base and sharing culture that the BIA advocates for in this update, as well as the required trust in data sharing and usage called upon in the Department of Health and Social Care’s ‘Data Saves Lives Strategy’. Ultimately TechBio will help us move from symptom management towards slowing, stopping and even curing some of the most complex diseases through the development of data-driven, digitally-enabled therapies that deliver better outcomes for patients.
Understanding TechBio
A year ago, we've launched “TechBio: How data-driven life sciences companies are transforming drug discovery and patient care” to define, explore and create consensus around the growing group of companies working in this area of the industry. Previous labels for these companies, including digital health or data technology, failed to capture the breadth of work these companies are carrying out at the intersection of technology and biotechnology.
Through primary research with companies and sector experts, we landed on the term ‘TechBio’ by taking the ‘tech’ of technology and the ‘bio’ of biotechnology to group together these companies.This digitalisation of biology through the application of artificial intelligence (AI) and machine learning to data is enabling greater opportunities to standardise, automate and industrialise biological processes.

Policymakers are focusing on how public data can be shared securely and effectively so that researchers and TechBio companies can draw vital insights from it that will drive change in healthcare and drug discovery. The COVID-19 pandemic increased public interest in how health data can be collected and the benefits of using this data to rapidly develop vaccines, manage healthcare resources and repurpose existing drugs.
People have seen that donating and sharing their data can play a direct role in improving health outcomes, similar to donating blood. Despite game-changing advancements in data-driven life sciences during the pandemic, the public still have concerns over how their data is managed and shared. Building trust and maintaining transparency continue to be vital to reassure people that their data is being shared and used responsibly and for positive purposes and to encourage them to continue sharing it.
Developments in data sharing
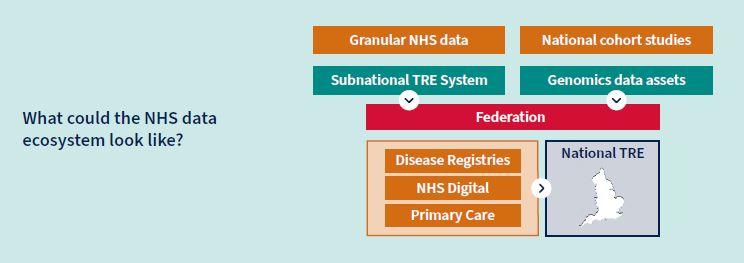
Data is the lifeblood of TechBio companies, and how that data can be shared in a secure and effective way through trusted research environments continues to be a hot topic. A trusted research environment (TRE) is a secure environment that researchers enter in order to work on the data remotely, rather than downloading it onto their own local machine. Users can extract and download the answers from their analyses – such as results tables, or graphs – but individual patients’ data always stays within the secure environment.
The UK has a unique global advantage with the NHS as a source of comprehensive health data for the population. The NHS Vision states that it will: “by 2025 have a world leading NHS-wide health data research infrastructure that enhances patient care, sustains the NHS and supports innovation.” To make this vision a reality, in March 2022 the government committed £200 million to better facilitate access to NHS Health data. The £200 million investment aims to make research-ready data available in a streamlined, secure way through secure data environments (SDEs). A set of national and subnational SDEs will become the default route for accessing NHS data for research and innovation. The government’s Data Saves Lives strategy commits to an accreditation scheme and full technical specification for the SDEs, drawing on industry best practice. This should both streamline access for data users and provide a higher level of security and transparency for the public on data sharing.
Some recent progress in adopting SDEs includes:
- NHS Digital’s (NHSD) national SDE
NHS Digital is currently piloting a national secure data environment, which provides approved researchers from trusted organisations with timely and secure access to NHS health and social care data.
- Sub-national SDE
The network of sub-national SDEs is being piloted in four locations to scope and define how NHS-owned secure data environments might work best at a regional level.
- Federated data platform
NHS England intends to procure a federated data platform to maintain the valuable data connectivity developed over the past few years using the COVID-19 data platform.
Case studies
The following four case studies illustrate just some of the innovation coming out of UK data-driven life sciences companies:

Constructive Bio is headquartered in Cambridge since 2022. Interview with CEO, Ola Wlodek and Head of R&D, Daniel de la Torre
What does the company do?
Constructive Bio is recoding biology to design novel organisms, molecules and products. Their platform reprograms the fundamental protein translation process, turning cells into bio-renewable factories for producing novel molecules that nature could not make before. They are also rewriting new genomes from scratch to engineer unnatural polymers.
"Nature has this amazing system for creating very complex polymers in a completely programmable manner. DNA contains the set of instructions that cells use to make proteins, which are polymers, built of 20 different amino acid building blocks. We are repurposing this natural system to create new classes of polymers where we can genetically programme the sequence, order and identity of novel monomer building blocks, to discover new classes of therapeutics and biomaterials." - Daniel de la Torre, Head of R&D
How does the technology work and how will it be used?
Constructive Bio are pioneering approaches for building entirely synthetic genomes and for reprogramming the genetic code of living organisms for programmable polymer synthesis.
- Genome synthesis: Constructive Bio’s REXER platform can assemble synthetic DNA at the megabase scale and implement it into biological systems, building entire genomes from scratch. The ability to rewrite entire genomes allows them to radically change the genetic code and equips the new synthetic cells with the necessary genetic software to enable new properties.
“Technology such as Crispr can do this to individual genes – however our platform aims to be able to rewrite genomes at large scale. Recoded genomes are valuable tools and Constructive Bio is already creating phageresistant bacterial strains that can be used in different bioprocesses, such as the production of plasmid DNA for mRNA vaccines.” - Ola Wlodek, CEO
- Encoded polymer synthesis: Constructive Bio’s polymer encoding system will explore unchartered chemical space, generating molecules and products with novel and valuable properties. The company is using DNA as a programming language for encoding novel polymers, and can program the installation of complex, unnatural functionalities at defined positions within proteins. Constructive Bio is aiming to work with companies who require polymers for specific needs and will use their process to create cleaner and greener polymers than those formed through chemical synthesis. Examples of items that could be created include industrial anti-bacterial coatings or polyhydroxy-acids that are used in beauty products.
The future
"Possibly the most exciting thing from a TechBio perspective is that by developing our platform, we will also be able to learn the rules of how to write genomes of the future. We want to become a lead producer of unnatural polymer-making strains to create products where there is an industrial need, and help unleash a new era in the bioeconomy." - Ola Wlodek, CEO

MultiOmic is headquartered in London since 2021. Interview with CEO Robert Thong.
What does the company do?
MultiOmic applies artificial intelligence to create new precision treatments for metabolic syndrome-related conditions (“MetSyn) such as type 2 diabetes, chronic kidney disease, atherosclerotic cardiovascular disease, fatty liver disease and polycystic ovary syndrome.
“These conditions are all linked, patients often suffering from multiple conditions concurrently. There are hundreds of disease variants, each corresponding to a subgroup of patients experiencing a unique sequence of symptoms and medical complications at different levels of severity.” - Robert Thong, CEO
How does the technology work?
MultiOmic’s MOHSAIC® platform is the first MetSyn-optimised Artificial Intelligence Drug Discovery (AIDD) platform. Starting with patient bio-samples (blood, urine, etc.) and corresponding medical records sourced from hospitals and biobanks, the company generates omics data (genome, epigenome, proteome, metabolome) using their standardised process, on which they apply AI-enabled computational techniques to identify specific patient subgroups and their corresponding biomarkers and causal biological pathways. These findings are validated via systems biology simulation models and bespoke wet laboratory experiments to subsequently derive more efficacious treatments.
How will it be used?
MetSyn-related healthcare costs amounted to 1.9 trillion dollars globally in 2020 and forecast to rise to 5.5 trillion in 2040. These conditions currently have no cures, only symptomatic treatments. Between 2011 to 2020, only 7.8% of cardiovascular, endocrine or other metabolic drug candidates in clinical trials gained regulatory approval, a worsening trend compared to 2006 to 2015 (11%). MultiOmic aims to reverse this trend via precision medicine, where biomarkers are used to identify specific patients that would most benefit from a particular treatment. There are presently no MetSyn precision medicines. Whereas it has been proven in cancer and rare disease that precision medicines have more than double the probability of reaching the market than non-precision ones.
MultiOmic aims to generate unique treatment concepts for each patient subgroup its platform identifies. These treatments could include novel combinations of existing drugs; repositioning “failed” drugs on high responder patients; repositioning drugs from other indications; and/or working with partners to develop completely new drug molecules.
“MeSyn affects 1 in 3 adults including 1 in 2 over 60s, there is a huge unmet need. Despite having their symptoms treated, many patients still go on to develop fatal or seriously debilitating complications. It’s vital we understand the drivers for MetSyn and identify better treatments to tackle the underlying causes.” - Robert Thong, CEO
The future
Although initially focused on MetSyn, MultiOmic’s approach could be extended to other chronic multifactorial conditions including autoimmune diseases such as asthma or inflammatory bowel disease. The company is aiming to have repositioned drugs in clinical testing within the next 3-4 years and will be looking to partner with pharma/biotech companies and CROs.
 NonExomics is headquartered in Boston, US since 2021 and has subsidiary branches in Cambridge, UK and Chennai, India. Interview with CEO, CTO, and Co-founder, Sudhakaran Prabakaran
NonExomics is headquartered in Boston, US since 2021 and has subsidiary branches in Cambridge, UK and Chennai, India. Interview with CEO, CTO, and Co-founder, Sudhakaran Prabakaran
What does the company do?
Almost all current therapies target proteins that are made by two percent of the genome, known as the exome, but NonExomics has demonstrated and validated that the rest of the 98% of the human genome, called the nonexome, can also code and make proteins. Importantly, 80–90% of all disease mutations lie in the nonexome and hence to cure diseases it is important to understand the consequences of these mutations to the proteins discovered by NonExomics that are made in the nonexome.
How does the technology work?
"Think of genome like an iceberg – currently we only see the 2% of the proteins that are above sea level but what we are doing as a company is digging into the other 98% that lies below the water to see what those proteins are doing." - Sudhakaran Prabakaran, CEO, CTO and Co-founder
NonExomics have used their technology to query the entire human genome, transcriptome, and proteome and have identified around 250,000 never before discovered proteins. They have found these proteins are capable of forming structures involved in biological processes and are disrupted in a number of diseases. By applying their machine learning platform to patient-data at population scale, they have been able to identify more than 3,000 entirely novel targets strongly associated with 1,365 human diseases. These targets have been scientifically probed both at systemic and molecular level to investigate their druggability and the company plans to use this information to develop therapeutics to treat these diseases.
How will it be used?
One disease where NonExomics are hoping to identify new treatments is amyotrophic lateral sclerosis or ALS. This is a neurodegenerative disease and a number of genetic mutations that had previously been identified within the disease had been classified as unknown or benign, as people thought that these regions of the genome do not make proteins.
By applying their methodology to these regions of the genome, NonExomics has shown that the regions do actually make protein products. They have also discovered that mutations caused by the disease are creating a consequence to those proteins, such as prematurely fragmenting them or elongating them to completely disrupt their structure. NonExomics is now working to build a full understanding of ALS using this newly discovered data so they can then look at developing therapies to address mutations and treat the disease.
The future
NonExomics is already working with pharma companies to explore the druggable targets that it has identified and will be looking to licence out further drug targets in rare diseases and cancer to other pharma and biotech companies. Their longterm goal is to take their own targets through clinical trials.

BIOS Health is headquartered in Cambridge since 2015. Interview with Oliver Armitage, Chief Scientific Officer and Co-Founder
What does the company do?
BIOS is leveraging the nervous system to inform treatments and deliver therapies for chronic conditions. The company uses AI and machine learning technology to tap into the nervous system which acts as the controlling, regulatory and communication system for the body, connecting all organs to the brain and spinal cord.
“We can record and electrically stimulate the nervous system to understand both how a disease or an organ system is progressing, and how neural regulation is being done by the brain. We then use this information to intervene with drugs or devices that can help bring the organ back into a healthier state.” - Oliver Armitage, Chief Scientific Officer and Co-Founder
How does the technology work?
BIOS are specialists in collecting neural data and do it in a number of ways. Data can be taken directly from a patient either with an implant that transmits data from the nerve to the device, by minimally invasive collection through a bedside box, or proxies of neural data can be collected through wearable devices. Once the company has collected the data it then uses two sets of AI and machine learning techniques. The first decodes the data coming from the nervous system and then a second set identifies recommendations for treatment and intervention based on the neural code. Oliver said: “By understanding the messaging from the nervous system and being able to decode that language and ‘speak nerve’, we can link up what's going on within whole subsets of patients and identify new treatment options.”
How will it be used?
BIOS’ solution is to build ‘neural digital therapies’. A neural digital therapy is a precision therapy developed using insights from neural recordings. These can range from precision bioelectronics and companion diagnostics to neural biomarker assisted drug development. In 2020, BIOS launched the Autonomic Therapy Initiative (ATI) programme to realise a neural digital therapy for cardiovascular diseases that are responsible for a third of all deaths globally. The ATI programme leverages the BIOS approach of unique digital neural biomarkers and machine learning AI to offer a level of precision unseen before in healthcare. The programme will deliver personalised, real-time treatment recommendations, shaping the design of new therapies and even replace current drugs with computer generated neural signals.
Oliver said: “Genomics data has been used by therapeutics companies to create medicines based on genome precision. What we have discovered is that the nervous system gives you the ability to target precision treatments on all of the chronic conditions that genetics aren’t as good at. Neural is the next wave of precision medicine”
The Future
BIOS is working to bring this whole new field of Neural Digital Therapies forward to enable neural based precision medicine for chronic diseases where it would be deeply impactful. Oliver said: “We want to work with people with deep disease expertise, in more disease areas like inflammation, diabetes, respiratory, and neurological conditions, to bring forward the greater precision treatments that neural biomarkers and neural data allow us to do to those disease areas.”
The Future
Sector skills – Securing talent that techbio companies need to thrive in the UK
TechBio companies face double the challenge when it comes to securing the talent needed in order to thrive and grow as they work across biotechnology and technology. Due to launch next year, the BIA is planning a national digital advertising campaign to help address the large gaps in advanced digital and data-driven skills for TechBio companies. The aim of the campaign is to help industry attract and inspire next-generation digital talent from a range of IT-related or IT infrastructure job titles and from AI, data and machine learning skills/courses including software engineers, data scientists, digital innovators and computer scientists.
The campaign will raise awareness of the fantastic career opportunities there are in biotech, in particular in TechBio, which is a rapidly growing area of the sector offering exciting career progression alongside the opportunity to make a difference to people’s lives, their health and the planet.
The BIA are already supporting to ensure industry apprenticeships are made available, for example, we’ve helped launch data analyst and bioinformatics apprentice opportunities.
Digital Talent Survey: tell us what you think
To ensure the digital skills campaign attracts the talent the industry needs, we would like to hear your thoughts on:
- Where do you currently hire data scientists and digital talent from?
- What computational skills does your organisation need the most now and in the future?
- What level of skills/stage of career (i.e. apprentices, graduates, doctoral, early career, career transition) are you looking to recruit?
- Is your search focussed on the UK or also looking at attracting international talent?
Building momentum behind TechBio
-
Be part of the BIA TechBio LinkedIn group to connect and explore
-
Join the BIA and be part of our growing TechBio community
The BIA continues to act as a hub for all things TechBio and to ensure that the organisation is delivering the support and advocacy that TechBio companies need it has established a special community. The group is led by BIA Senior Policy and Public Affairs Manager, Emma Lawrence – for more information or to join the group, please email [email protected]
You can read more on TechBio, including a soon to be published position paper on what industry needs from the new SDE ecosystem on the BIA website.
.png)
.png)




