Foreword
This report shows that the ecosystem that governs access – from regulation through appraisal to commissioning and adoption – has not kept pace with advances in scientific innovation.
In the survey undertaken to inform this report, stakeholders across industry, the NHS, academia and patient organisations told us the same thing: the UK’s rare disease access pathway is fragmented. Evidence expectations vary across different parts of the system. Appraisal methods often struggle to reflect small populations or non-traditional endpoints. Early access routes are inconsistently used and not always commercially viable. And even when reimbursement is secured, adoption across the NHS remains uneven and lacks national leadership to drive accountability.
Other countries have acted decisively to address these challenges. Amongst others, Austria, France, Germany and Spain have created more flexible approaches that enable earlier, predictable access while maintaining public accountability. If the UK is to remain competitive as a launch market and to truly deliver for people living with rare diseases, it too must do the same.
The solutions outlined in this report offer a practical way forward: earlier and more structured dialogue between regulators, Health Technology Assessment (HTA) bodies, and companies; a more proportionate appraisal and access pathway for small-population therapies; stronger adoption mechanisms through specialist hubs and clearer clinical leadership; and a renewed national strategy to drive reform over the short, medium and long term.
Most importantly, these solutions focus on what matters: ensuring that innovation translates into real and timely benefits for patients. By embedding equity at each stage of the pathway, they help ensure that access is determined by clinical need – not by geography, condition type or system variation.
The BIA, working through its Rare Disease Industry Group (RDIG), is committed to helping deliver these changes. With the right reforms, the UK can turn its scientific excellence into an access system that is equitable, faster and fit for the future – and ensure that no person living with a rare disease, nor their families, waits longer than necessary to receive the treatments they need.
Lawrence Tallon, CEO of the Medicines and Healthcare products Regulatory Agency (MHRA):
Nick Meade, CEO of Genetic Alliance UK:
Executive summary
The UK enjoys a reputation as having one of the most advanced and trusted healthcare systems in the world. Yet, when it comes to patient access to rare disease medicines, the system still lacks the flexibility and pragmatism needed to recognise innovation and ensure patients benefit from it.
Today, the pathway through which rare disease medicines move from regulatory approval to patient access is, all too often, fragmented and unpredictable. This fragmentation creates inequitable access across conditions, regions and patient groups, despite the UK’s strong scientific and clinical foundations.
While individual components – e.g. the Medicines and Healthcare products Regulatory Agency’s (MHRA’s) efforts to streamline regulatory approval processes, the National Institute for Health and Care Excellence’s (NICE’s) rigour in HTAs, and the NHS’s capacity to deliver an advanced standard of care – each perform strongly in their own right, they do not consistently operate effectively as an interconnected ecosystem.
This is reflected in the BIA’s Health Ecosystem Stakeholder Survey, conducted amongst rare disease stakeholders, to inform the development of this report. It is also evident in the 2025 Association of the British Pharmaceutical Industry (ABPI)-BIA Rare Disease Member Survey, which found companies frequently encounter duplicated evidence requirements, inconsistent expectations between regulatory and HTA bodies, and limited flexibility within formal appraisal frameworks.
For patients and clinicians, these fragmentations result in slow and uneven access to promising new therapies. Despite ongoing efforts to evolve existing processes, the system, as a whole, remains poorly configured to accommodate the realities of rare disease drug development: small populations, limited comparators, and non-traditional clinical endpoints.
Other countries – including Austria, France, Germany and Spain – have adopted more proportionate and flexible approaches, and are already seeing faster access, greater investment and stronger links between innovation and uptake. To retain its leadership in life sciences – and to turn discovery into delivery – the UK must act decisively, and it must act now.
This report builds on a growing body of evidence calling for reform. It draws on and complements the BIA’s 2023 report with PwC, which compared how the UK performs internationally on rare disease access; LifeArc’s 2025 report Accelerating R&D for Rare Disease in the UK, which identified key actions to drive forward research into new rare disease treatments and technologies; and PCD Research’s new Blueprint for accelerating rare disease innovation and clinical trials in the UK, which sets out the opportunity for the UK to unlock a paradigm shift in rare disease research.
Together, these reports point to a clear and shared conclusion: the UK has the scientific strength and institutional foundations to lead in rare disease innovation, but doing so requires a more connected, proportionate and outcomes-focused approach that embeds equity throughout the access pathway.
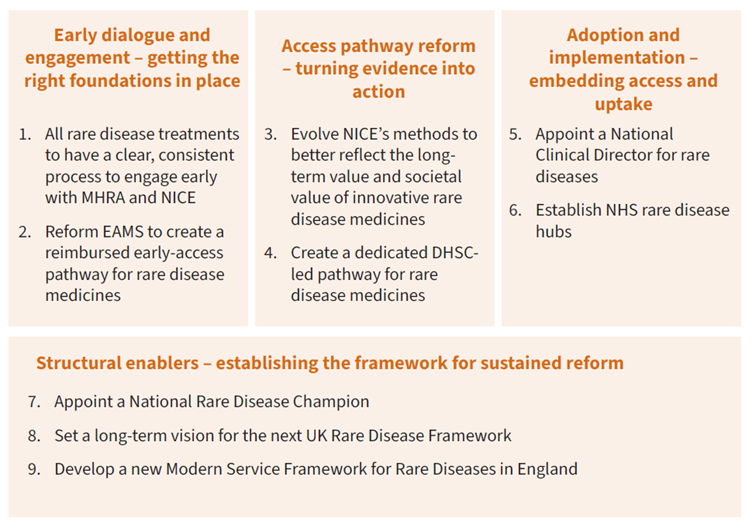
Building on these contributions, this report sets out a practical framework for reform to ensure that patients in the UK can benefit from this innovation. It identifies four areas where targeted change would deliver the greatest impact:
- Early dialogue and engagement between regulators, NICE and companies to align evidence expectations and bridge licensing and access.
- Access pathway reform to ensure the full value of innovative medicines are recognised.
- Adoption and implementation measures to drive system coordination, promote uptake and deliver stronger real-world data and clear accountability for implementation.
- Structural enablers to embed visible national leadership and align delivery across government, the NHS and the life sciences sector.
Taken together, these solutions would create – and maintain – a more equitable, faster and more predictable access pathway; one that delivers for patients, strengthens the NHS, and ultimately takes the UK’s potential as a global leader in rare diseases from innovation to impact.
Summary of proposed solutions:
Introduction
People living with rare diseases face high levels of unmet need
Across the UK, over 3.5 million people – approximately one in 17 people – live with a rare disease. While each condition may affect only a small number of individuals, the collective impact of these conditions on the health and care systems, on families and carers, and on wider society is substantial. Approximately 70% of these conditions begin in childhood, and many are chronic, life-limiting and progressive.
Despite major advances in genomics, data science and precision medicine, fewer than 5% of all recognised rare diseases currently have a licensed treatment. Patients and their families therefore face long diagnostic journeys, limited information and few or no options for treatment.
Where treatments do exist, however, the picture is notably different: diagnostic times are significantly shorter. Across Europe, the time to diagnosis is around four times faster for rare diseases with an available treatment than for those without. This demonstrates a virtuous cycle in which greater medical awareness and therapy availability support earlier diagnosis, enabling timelier and better-adapted care.
The UK’s limited provision of newborn screening exacerbates this diagnostic challenge. The NHS Newborn Blood Spot (NBS) Programme currently only screens for ten rare conditions – among the lowest in Europe – and the process of adding new conditions can take years. By contrast, several European countries routinely screen for more conditions – nearly five times as many in the case of Italy. The limited scope of UK screening constrains early diagnosis and timely referral into specialist care – both of which are vital when treatment windows are short and when clinical evidence depends on early intervention.
Yet even after diagnosis, many patients and families encounter a fragmented system of care. Specialist expertise for rare diseases is concentrated in a limited number of centres, referral pathways are inconsistent, and coordination between services remains weak. The absence of a defined national structure to link diagnosis, treatment and data collection means that opportunities for earlier intervention and shared learning are often missed.
This fragmentation extends to how the uptake of new treatments are adopted, with access to approved medicines varying significantly across regions depending on local commissioning capacity and clinical readiness. Recent analysis by the House of Commons Library highlights this persistent variation in the uptake of NICE-recommended medicines across England, reflecting broader challenges in translating innovation into consistent national practice.
This lack of coordination not only delays access to appropriate care, but also constrains the NHS’s ability to generate the high-quality, real-world evidence needed to inform commissioning, appraisal and policy.
The cost of inaction
The consequences of slow or inconsistent access fall heavily on patients, families, and the public purse. Although precise estimates are limited, a new report from PCD Research presents fresh analysis on this “failure to act”.
Headline findings include:
- £340 million – Annual NHS cost of delayed diagnosis.
- £3.5 billion – Annual local authority social care expenditure.
- £4.7 billion – Annual disability and welfare benefits.
- £14.9 billion – Estimated annual loss in tax revenue due to reduced workforce participation.
- 46% of parents of disabled children report that caring responsibilities affect employment; one in five leave work entirely.
A system not configured for rare disease realities
Unlike therapies for more common conditions, rare disease medicines face unique barriers across development, appraisal, and adoption. By definition, rare disease therapies target small populations with limited clinical precedent or established endpoints. Evidence, therefore, often relies on single-arm studies, surrogate outcomes, or real-world data. While regulators have made steps to support earlier approvals under uncertainty, downstream appraisal and reimbursement processes have been slower to adapt.
In England and Wales, most rare disease medicines are assessed through NICE’s Single Technology Appraisal (STA) process, which was not specifically designed to manage high uncertainty or very small patient populations. The Highly Specialised Technologies (HST) route provides an alternative, but its criteria are tightly defined, and many therapies and indications fall outside its scope.
Recent commitments to increase NICE’s STA cost-effectiveness thresholds and implement a new value set for measuring the impact of new medicines are a welcome signal that the system is beginning to evolve. However, for rare disease medicines, where evidence constraints, small populations and long-term societal impacts sit outside conventional appraisal metrics, these reforms will only deliver meaningful improvement if they are designed in ways that genuinely work for rarity. Broader value assessment must therefore be implemented with explicit recognition of the distinct evidentiary realities of rare conditions.
Recent data reinforces this challenge. In 2023/24, 18 orphan drugs were assessed under NICE’s STA process (six recommended) and seven through HST (five recommended). In 2024/25, 15 orphan drugs were routed through STA (11 approved) and only one through HST, which was not recommended.
This pattern underscores a structural issue: while the UK has mechanisms to assess rare disease medicines, they are not configured for the realities of small populations, limited evidence, and high unmet need.
Findings from the 2025 ABPI-BIA Rare Disease Member Survey reinforce this. Companies cited the restrictive use of the HST pathway and the rigidity of STA methods as key reasons why many orphan drugs are not submitted for NICE evaluation, despite MHRA approval.
Internationally, this is reflected in the UK’s declining position on access to rare disease medicines, with the European Federation of Pharmaceutical Industries and Associations (EFPIA)’s Waiting to Access Innovative Therapies (W.A.I.T.) Indicator Survey finding that the UK ranked second in Europe for orphan drug availability as of 2018, but has since declined, with England now ranking tenth, and Scotland twelfth, for the availability of non-oncology orphan drugs, according to the 2025 dataset.
These findings also speak to a broader strategic question facing governments in the UK and internationally: how to sustainably fund innovative medicines, particularly in rare diseases.
Emerging analyses show that spending on Orphan Medicinal Products (OMPs) is unlikely to threaten the sustainability of national pharmaceutical budgets. Instead, differences in OMP uptake between countries appear driven less by economic capacity and more by political prioritisation and policy choices.
This highlights that current access barriers are structural rather than fiscal – and that with the right policy frameworks, it is possible to support earlier, more equitable access without compromising budgetary discipline.
Enduring challenges and a changing regulatory context
The scientific and commercial challenges of developing rare disease medicines are well known. Small patient populations limit trial size and statistical power; the absence of natural-history data complicates the evaluation of long-term benefit. For developers, these challenges drive up development costs and risk, sometimes leaving no commercially viable route to reimbursement. For patients, the result is delayed or inconsistent access to therapies, even when those treatments are clinically approved.
The UK has long recognised the need for proportionate approaches outside standard HTA pathways, using mechanisms such as specialised commissioning via the Clinical Priorities Advisory Group (CPAG), Individual Funding Requests (IFRs), and early managed-access arrangements. While some remain in use, many predate more structured processes such as the HST and the Cancer Drugs Fund (CDF). Their continued relevance demonstrates an enduring recognition that small-population therapies often require bespoke decision-making.
More recently, this need has been acknowledged at a regulatory level. The MHRA’s Rare Therapies and UK Regulatory Considerations paper, published in November 2025, sets out proposals for a new, dedicated regulatory pathway for rare therapies to make it quicker and easier to test, manufacture and approve treatments in the UK. They represent a positive statement of intent to position the UK as a global leader in rare disease regulation. However, their impact will depend on parallel progress across appraisal, reimbursement and commissioning so that regulatory flexibility translates into earlier and broader patient access.
Learning from international comparators
Many European countries have embedded mechanisms to accelerate access to rare disease therapies while maintaining fiscal discipline. As highlighted in the 2023 BIA–PwC report, Evaluating patient access to rare disease treatments, these systems share core features: clear eligibility criteria, proportionate evidence requirements, and time-bound reassessment.
Austria: Austria’s rare disease system delivers some of the fastest and broadest access to orphan medicines in Europe, with over 90% of European Medicines Agency (EMA) approved orphan products available to patients. In part, this is enabled by the Erstattungskodex (Reimbursement Code), which enables new medicines to be listed and reimbursed rapidly following EMA authorisation, with pricing negotiations handled in parallel. Whilst not automatic, this process allows patients to access reimbursed treatments significantly faster than in most European countries. Austria’s participation in the Beneluxa Initiative since 2016 has reinforced this framework, combining joint horizon scanning, collaborative price negotiations and early dialogue with manufacturers to align system readiness before approval. This is complemented by the 2015 National Plan for Rare Diseases, which established a National Coordination Centre and a network of designated centres of expertise to connect research, clinical care and data infrastructure.
France: France’s Autorisation d’Accès Précoce (AAP) offers an established route for patients to access innovative medicines, including orphan products, before full market authorisation. The scheme provides clear procedural timelines (applications processed within three months), temporary free pricing with a payback mechanism if the final price is lower, and access to treatments with serious unmet need. Between 2018 and 2023, 221 products entered the AAP, one-fifth of which were orphan medicines – representing 39% of all EU-approved orphan drugs during that period. The average 77-day processing time highlights France’s commitment to timely, structured patient access to innovative medicines.
Germany: Germany’s system allows new medicines to be marketed and reimbursed immediately after regulatory approval, removing a major barrier to patient access by ensuring patients benefit quickly from innovation. Manufacturers can freely set prices for six months while the Federal Joint Committee (G-BA) assesses the medicine’s additional benefit compared to existing therapies. A payback mechanism applies if the final negotiated price is lower. In cases of dispute, an independent arbitration board determines a binding price. Unlike France’s scheme, the German model applies to all new innovative medicines, not only those addressing unmet needs, providing a universal, predictable framework that balances early access with cost control.
Spain: Spain operates an early-access framework centred on the Autorización de Uso Compasivo (AUC) and, more recently, the Autorización Temporal de Comercialización (ATC), which together enable patient access to innovative or orphan medicines prior to formal national reimbursement. These routes allow the use of medicines that have received EMA marketing authorisation but are awaiting national pricing and reimbursement decisions. In parallel, Spain has increasingly deployed payment-by-results and risk-sharing agreements at a regional level, allowing conditional reimbursement linked to clinical outcomes.
Developers of rare disease treatments are choosing not to market their products in the UK because of the low chances of reimbursement with the current HTA assessment processes, with many also terminating their HTA applications. - Survey respondent, Patient Group / Charity
The UK and devolved governments have similarly put in place some steps to strengthen rare disease policy and infrastructure
The 2021 UK Rare Diseases Framework established four national priorities: faster diagnosis, improved awareness among healthcare professionals, better coordination of care and fairer access to treatment. Each UK nation has subsequently published action plans to implement these priorities, supported by regular progress updates to help maintain momentum.
Complementary initiatives reinforce this direction, across each of the four nations of the UK, with the publication of the Life Sciences Sector Plan (LSSP) and 10-Year Health Plan (10-YHP) being amongst some of the most significant recent developments from a Westminster government policy perspective (see box below). Together, these developments offer a strong policy foundation from which to build and achieve progress. The challenge now is to translate this momentum into measurable improvement in patient access.
Rare disease policy context in England
The UK’s health and life sciences agenda is largely influenced by three interlinked strategies that set the context for rare disease reform. The LSSP underscores the UK’s strategic commitment to transform the sector into the leading life sciences economy in Europe by 2030, and the third most important globally by 2035, through supporting scientific innovation.
The 10-YHP commits to creating fundamental reform within the NHS to deliver faster diagnosis and fairer access across all disease areas via shifts from hospitals to community care, treatment to prevention, and analogue to digital systems. Rare diseases feature within the prevention pillar, with commitments to expanding genomic testing at birth and to re-evaluate clinical pathways to enable faster diagnosis and more personalised care. This includes the Generation Study, a landmark programme, backed by £105 million in funding, to pilot the use of whole-genome sequencing in newborns which will continue recruiting and sequencing the genomes of 100,000 newborn babies with parents’ consent to detect hundreds of rare genetic conditions early in life and inform future prevention and treatment strategies.
Building on these, the England Rare Diseases Action Plan 2025 introduces new measures: expanding NHS Genomic Networks of Excellence, embedding multi-system disorder clinics, piloting n-of-1 therapy frameworks, and implementing clinical-trial and digital-genomics reforms to reduce diagnostic delays and widen access.
The MHRA has also set out proposals to strengthen regulatory support for rare therapies through its 2025 paper, Rare Therapies and UK Regulatory Considerations. This document outlines a programme of major reform, including the creation of a new, dedicated regulatory pathway for rare therapies to make it quicker and easier to test, manufacture and approve these treatments in the UK.
Despite progress, the UK continues to trail its peers on access and adoption
The UK remains a global leader in biomedical research, but this leadership is not yet matched by performance on patient access. While the pace of scientific discovery has accelerated, the frameworks that govern regulatory approval, appraisal and reimbursement have evolved more slowly.
The UK Government’s Life Sciences Competitiveness Indicators (published in 2024) show that the proportion of new medicines available in England compared with those approved by the European Medicines Agency (EMA) has fallen from 72% in 2016–2019 to 56% in 2019–2022, thereby highlighting the continued challenges in securing timely access to innovation for patients.
Recent international comparisons underline the scale of the challenge. Only 50% of non-oncology orphan drugs approved by the EMA between 2020-2023 were available to patients in England by 5 January 2025 (46% in Scotland), compared to 85% in Germany, 74% in Italy, 67% in France, and 61% in Spain. This decline illustrates a widening gap between the UK’s research excellence and its ability to translate innovation into patient access. Findings from the BIA’s Health Ecosystem Stakeholder Survey, detailed in the next section of this report, reinforce this picture.
The opportunity now is to connect science, policy and delivery into a more defined access pathway
The UK’s rare disease challenge does not lie in the quality of its science, but in the coordination of its systems. Despite world-class research, too many innovations stall between discovery and delivery.
Building on insight from stakeholders across the health and life sciences ecosystem, the following sections of this report propose a practical framework for achieving this ambition of greater integration. It maps the rare disease access journey – from discovery through approval, appraisal, reimbursement and adoption – identifying where targeted changes can help support better patient access to innovative rare disease medicines.
Survey findings
BIA Health Ecosystem Stakeholder Survey findings
To inform the development of the solutions detailed in this report, the BIA RDIG conducted a targeted survey of cross-ecosystem stakeholders. The survey brought together 34 senior stakeholders from across government, arm’s-length bodies, the NHS, academia, patient organisations, and industry. Each participant had direct experience of the UK’s rare disease policy environment, regulatory processes, or patient-access pathways. Together, they provide a broad and informed snapshot of how the system is working in practice.
Across this diverse group, a consistent message emerged: the UK has world-class scientific and regulatory foundations, but the systems that govern access have not kept pace with the realities of rare disease innovation. The pathway from approval to adoption was described as fragmented and unpredictable, with duplication of effort, inconsistent expectations, and slow progression from regulatory approval to patient use.
Headline BIA RDIG Health Ecosystem survey findings:
- Nearly two-thirds (64%) believe access to innovative medicines for UK rare disease patients to be poor. Only 12% say the same is true for patients with non-rare diseases.
- More perceive rare disease patients’ access to innovative medicines is worse compared to five years’ ago (36%) than believe it has improved (21%).
- Only 9% say that UK rare disease patients have better access to innovative medicines compared to countries with comparable levels of development.
- A majority (56%) also believe the way medicines are valued and appraised is either ‘not working well’ or are ‘working very badly’.
- Well over half (60%) of respondents believe payment models for rare diseases in the UK are either ‘not working well’ or are ‘working very badly’.
Similarly, more than 70% believed that the UK performs worse than peer countries such as Germany or France. Respondents pointed to the same structural barriers throughout: delayed engagement between developers and decision makers, appraisal criteria unsuited to small-population evidence, and limited NHS readiness to adopt new therapies once approved.
These findings are mirrored in company sentiment
The 2025 ABPI–BIA Rare Disease Member Survey found that challenges around demonstrating cost-effectiveness, limited flexibility in NICE’s STA route, restrictive eligibility for routing to the HST pathway, and high Voluntary Scheme for Branded Medicines Pricing, Access and Growth (VPAG) rebate rates are shaping launch decisions for innovative rare disease products.
This industry survey found that, of the 53 UK marketing authorisation applications and variation applications for rare disease indications reported in the survey since 2020, 91% of these applications were submitted to the MHRA only after EMA approval, with several companies choosing not to pursue UK launch at all due to uncertainty regarding subsequent access and reimbursement. Others achieved authorisation but were not submitted to NICE for appraisal, while some appraisals were withdrawn or terminated; all of which have since become available to patients in other major European markets.
Taken together, these survey findings point to a system that has world-class scientific capability but outdated access architecture. They underline the need for a more coordinated and adaptive approach – one that aligns evidence requirements earlier, modernises appraisal and payment frameworks, and provides predictable pathways for promising therapies.
Solutions
Solutions to improve patient access to rare disease medicines
Improving access to medicines for rare and ultra-rare diseases depends not on a single reform, or a silver bullet, but on how effectively the system connects across the full pathway – from early development to adoption in clinical practice.
As set out in the preceding sections, all too often, biopharmaceutical companies encounter variable expectations, duplicated requirements, and uncertainty over how evidence generated in one stage will be interpreted in the next. The result is a process that can feel fragmented and unpredictable, even though certain individual parts may perform well.
Stakeholder feedback from the BIA Health Ecosystem Survey points to a clear way forward. What is needed is a more linear and predictable access journey: one that sets evidence expectations early, bridges approval and adoption, and provides structured ways to manage uncertainty while patients benefit.
This section sets out how this aspiration can be realised. By making engagement earlier, more predictable, and more proportionate to the realities of rare disease, we can reduce uncertainty for all parties and ensure that promising treatments reach patients more quickly.
From innovation to impact: how the rare disease access pathway can be improved
To move from innovation to real patient impact, the rare disease access pathway needs to shift from today’s fragmented, unpredictable journey to a more connected and proportionate model.
By aligning expectations early, tightening the link between approval and adoption, and creating clearer, better-coordinated routes through the system, we can build an access pathway that consistently moves promising treatments to patients faster and more equitably.
|
Phase |
Characteristics of the current rare disease access pathway |
Characteristics of a reformed rare disease access pathway |
|
Early dialogue |
|
|
|
Access pathway reform |
|
|
|
Adoption and implementation |
|
|
|
Structural enablers |
|
|
Early dialogue and engagement – getting the right foundations in place
Early and open dialogue between regulators, HTA bodies and companies is essential to building a shared understanding of evidence requirements and possibilities, to ensure that promising rare disease treatments can move smoothly from licensing to patient access.
For rare disease therapies, early dialogue matters more than anywhere else. Many of these conditions are unfamiliar to regulators and HTA bodies, and the supporting evidence base is often limited. Developers, therefore, rely on alternative or non-traditional endpoints to demonstrate clinical benefit and capture a therapy’s potential impact. Small and dispersed patient populations, few comparators, and the practical challenges of running conventional trials make evidence generation inherently complex, thereby reinforcing the importance of clear, coordinated engagement across the system from the outset.
This problem is well recognised. Respondents to the BIA stakeholder survey identified data and evidence as one of the most important enablers of access, with nearly 50% ranking it either first or second out of four enablers. Discussions at BIA RDIG workshops confirmed why: when evidence expectations are unclear or unrealistic at the outset, every subsequent step in the process is affected. International examples show that a more structured approach is possible.
Respondents saw value and appraisal and data and evidence themes as highly important for enabling better patient access to innovative rare disease medicines.
BIA Health Ecosystem Stakeholder Survey – Theme definitions
- Data and evidence – Refers to how the UK system defines, accepts, and makes use of evidence for rare disease medicines. This includes the process for determining what types of evidence can be used, how data from different sources and geographies is treated, and how evidence can be collected and updated over time to inform access decisions
- Value and appraisal – Refers to how the value of rare disease medicines is assessed in the UK. This includes the methods and criteria used to determine benefits, risks and costs, and uncertainties inherent with small patient populations, and whether these approaches are reflective of the broader context.
- Payment models – Refers to how rare disease medicines are paid for and funded through the NHS, including the mechanisms for providing access, balancing risk, and affordability, and how these arrangements can be adapted to support earlier access.
- Adoption and implementation – Refers to how rare disease medicines are introduced and scaled across the NHS, including how prepared services are, consistency across regions and how patients are supported to access them quickly and equitably.
The UK has already laid important groundwork. Mechanisms such as the Innovative Licensing and Access Pathway (ILAP), joint MHRA–NICE scientific advice, and Early Access to Medicines Scheme (EAMS) offer valuable platforms for early engagement and managed access. Yet, their use remains uneven, reflecting both the absence of a consistent, system-wide approach and features of early-access schemes that limit routine uptake for very small populations.
Against this backdrop, embedding these tools within a more structured and predictable framework would deliver greater benefit.
Making early engagement a routine rather than discretionary feature of development would improve alignment between regulatory and appraisal processes, reduce uncertainty, and help ensure that evidence expectations are clear from the outset. A genuinely coordinated model of early dialogue would create firmer foundations for the rest of the access pathway, enabling promising treatments to move more smoothly from development to patient use.
-
All rare disease treatments to have a clear, consistent process to engage early with MHRA and NICE
A formalised, by default, process for joint MHRA–NICE advice should be introduced for all rare disease medicines. This would provide developers of innovative rare disease medicines with early, coordinated guidance on the evidence standards required for both regulatory and Health Technology Assessment (HTA). Engagement should explicitly cover the appropriate use and expanded acceptance of non-traditional evidence. This should include real-world data, patient-reported outcomes, digital endpoints, and evidence from adaptive, platform or single-arm studies, alongside clear alignment vis-à-vis expectations for post-licensing data collection.
By embedding this dialogue early in clinical development, regulators, assessors and companies can identify and address evidentiary gaps before submission. This would reduce uncertainty at appraisal, shorten timelines, and give all parties greater confidence that promising therapies are progressing through a coherent, joined-up system.
-
Reform EAMS to create a reimbursed early-access pathway for rare disease medicines
EAMS has been an important tool for enabling earlier access to treatments addressing high unmet need, but its current design limits its impact for rare diseases. Companies frequently cite the absence of reimbursement, administrative complexity, and uncertainty over the transition to routine commissioning as barriers to engagement.
These findings are reflected in the 2025 ABPI-BIA Rare Disease Member Survey, which found that the lack of reimbursement was the primary reason companies were less likely to consider using EAMS for future rare disease products. Indeed, nine out of fourteen respondents reported having actively decided not to apply for EAMS, highlighting the free-of-charge supply requirement, the additional workload, and uncertainty about subsequent reimbursement as major deterrents.
Reforming EAMS to provide reimbursed early access for innovative rare disease medicines, underpinned by structured data collection requirements, would therefore make it a more effective bridge between regulatory approval and NHS use.
Real-world evidence gathered through such a pathway could directly inform NICE appraisal and managed-access decisions, supporting both earlier availability for patients and stronger evidence for payers. This would also bring the UK into closer alignment with other European markets – such as Austria, France, Germany and Spain where paid early-access or temporary reimbursement schemes have helped accelerate patient access while supporting evidence generation.
By embedding early and structured engagement as a core principle of the rare disease access framework, the UK can make better use of its existing strengths, provide clearer guidance to developers, and create a more predictable and collaborative pathway from innovation to access.
Access pathway reform – turning evidence into action
Securing marketing authorisation is only one milestone on the path to patient access. For rare disease therapies, the period that follows remains one of the most uncertain and protracted stages of the journey.
While all new medicines enter health technology assessment, most are channelled through NICE’s Single Technology Appraisal (STA) pathway – an assessment framework primarily designed for larger patient populations with greater access to comparative data. For rare disease medicines, this framework can create an imbalance between the available evidence and the evidentiary thresholds applied. The result is that potentially transformative and curative therapies, developed to treat small and severely affected populations, face long and unpredictable routes to reimbursement and ultimately delayed patient access.
The HST route was created to address this challenge, but its remit is narrow, and the number of eligible products remains limited. Hence, for many rare disease therapies, there is no tailored pathway between licensing and routine commissioning, and they fall between the gap of HST and STA.
Findings from the BIA’s Health Ecosystem Stakeholder Survey that have informed the development of this report echo this picture. As outlined above, nearly two-thirds (64%) of respondents described access to innovative medicines for rare disease patients as poor or very poor, while more than 70% said it was worse than in comparable markets (with European markets frequently cited here).
64% of BIA survey respondents described the current level of access to innovative medicines experienced by UK patients with rare diseases, as poor or very poor versus only 12% for non-rare patients.
In particular, respondents highlighted “duplicated evidence requirements”, “inconsistent expectations between regulators and HTA bodies”, and “limited flexibility within appraisal frameworks” as key barriers to faster access. As one participant observed, “the system continues to reward volume over rarity, even where clinical value is transformative.”
To restore predictability, England needs a more flexible, proportionate framework – one that gives companies clarity on where and how their medicines will be assessed, while ensuring that public funding continues to be allocated in a transparent and evidence-led way.
3. Evolve NICE’s methods to better reflect the long-term value and societal value of innovative rare disease medicines
For many innovative rare disease medicines, a key challenge inhibiting equitable patient access is challenging appraisal frameworks that are not suitably configured to recognise their full value. The current Quality-Adjusted Life Year (QALY) cost-effectiveness model, while rigorous, can only ever be as robust as the data that underpins it. For rare, progressive and paediatric conditions, that evidence is inherently constrained: patient populations are small, outcomes unfold over long timescales, and conventional measures of quality of life fail to capture incremental or developmental change.
Recently announced reforms to update NICE’s cost-effectiveness thresholds and introduce a new value set for valuing health-related quality of life for use alongside EQ-5D-5L are, therefore, welcome signals that the valuation framework is beginning to adapt. However, these changes will only improve access for rare disease medicines if broader approaches to value assessment evolve in parallel – explicitly reflecting rarity, the evidential constraints inherent to small populations, and the wider societal benefits these treatments can bring.
In the absence of such provisions, the QALY framework risks overstating precision while under-representing benefit. This structural imbalance means that many rare disease treatments struggle to demonstrate “value” in conventional terms, even when their clinical and societal impact is profound.
These medicines frequently deliver gains that reach far beyond direct clinical outcomes: fewer hospitalisations, reduced social-care dependence, improved quality of life for carers, and better educational and employment opportunities for patients. Yet such wider benefits are not captured within the existing parameters of the QALY approach.
Findings from the BIA’s Health Ecosystem Stakeholder Survey reinforce this picture.
Value and Appraisal – i.e. the approach used to assess the benefits, costs and wider impact of rare disease medicines within the UK health system – achieved the highest overall ranking among four themes that respondents were invited to prioritise when considering what was most important to ‘get right’ in order to enable access. When asked to rank these four themes 41% ranked Value and Appraisal first and a further 41% second. In qualitative input to the survey, respondents highlighted the need to “move beyond traditional cost-effectiveness approaches that cannot capture the long-term or societal impact of rare disease treatments” and noted that current methods “do not adequately reflect rarity or severity effectively.”
To ensure that value assessments keep pace with the realities of innovation, NICE should therefore continue to evolve its methodology in the long term so that it reflects the full spectrum of benefits that rare disease treatments can bring – not just to patients, but to families, communities, and the health system itself.
This means greater flexibility in how value is measured, allowing bespoke endpoints and health-measurement tools for rare diseases where standardised processes fail to reflect meaningful change; and a more systematic, transparent use of modifiers for rarity, severity and innovation so thresholds are proportionate to unmet need.
A clear framework for when and how such modifiers apply would give developers earlier predictability, reduce the risk that small-population therapies are disadvantaged by methodological constraints, and ensure that treatments with transformative or curative potential are assessed in a way that reflects their wider societal value.
4. Create a dedicated DHSC-led pathway for rare disease medicines
For those rare disease therapies, where a full cost-effectiveness-based HTA is not appropriate, given small patient numbers, immature data and limited comparators, and where they also fall outside of the restrictive criteria for access via HST, the government should establish a dedicated Department of Health and Social Care (DHSC)-led access pathway to complement NICE’s existing processes.
Under this model, DHSC would conduct value-based, budget-impact assessments rather than conventional cost-effectiveness appraisals, enabling proportionate and pragmatic decisions. With the planned transfer of NHS England responsibilities into DHSC, commercial negotiations could draw on the principles of the CPAG, but with clearer entry criteria, transparent decision-making and specific focus on rare disease medicines.
Recent MHRA signalling through its 2025 paper, Rare Therapies and UK Regulatory Considerations, reinforces the need for such a route. While the paper sets a more proportionate regulatory approach for small population indications, these benefits will only be realised if downstream appraisal and commissioning are equipped to receive these products. A DHSC-led pathway provides that counterpart.
This pathway could be supported in part through some reallocation of the £350 million annual allocation for the Innovative Medicines Fund (IMF) – a resource currently severely under-utilised (see pullout box below). To ensure this approach remains sustainable and continues to support conditional access where appropriate, funding arrangements should be reviewed regularly to reflect products entering and progressing through the pathway. This would help maintain a predictable mechanism for both rare disease treatments and other innovations.
Too many promising medicines stall between MHRA approval and NICE appraisal; the system simply isn’t built for rarity. - BIA survey respondent, clinician
Creating a dedicated rare disease access pathway, underpinned by real-world evidence requirements, clear eligibility criteria and proportionate evidence standards – co-developed by health system partners and industry – would help extend the UK’s ability to support earlier access for patients with rare conditions while ensuring that managed access routes operate effectively and remain appropriately funded.
By learning from existing processes while extending their scope and transparency, this pathway would enable timely, proportionate and predictable access for medicines that fall outside traditional HTA parameters.
Under-utilisation of the IMF
The IMF was first established in 2022 to extend the principles of the Cancer Drugs Fund to non-oncology medicines.
Backed by £350 million in annual ring-fenced funding, it is designed to support earlier, managed NHS access for promising treatments where further evidence was still required to confirm long-term value for money. In doing so, it aims to strike a balance between timely access, evidence generation and fiscal responsibility.
However, utilisation of the fund has been limited, with only £2 million being spent in the financial year ending 31 March 2024. Despite being created with the specific ambition of supporting patients with rare and genetic conditions, the fund has yet to provide a meaningful route to access for these treatments, thereby falling short of its original intent to fast-track innovative medicines for those with the greatest unmet need.
The findings from the 2025 ABPI-BIA Rare Disease Member Survey offer valuable insights on precisely why the IMF has remained significantly underused.
Companies reported that the current design of the IMF creates disproportionate financial risk for rare disease launches – particularly the requirement to continue supplying treatment free of charge if NICE issues a negative decision at the end of the managed access period. Respondents also highlighted that the five-year evidence-collection window is often too short to address uncertainties in rare conditions and that, in the absence of any meaningful risk-sharing mechanism, companies shoulder most of the risk.
Unlocking funding in the IMF to support a dedicated Rare Disease Access Fund would give it renewed purpose – transforming an underused instrument into a practical, sustainable mechanism for patient access to innovative treatments.
The risk should not be all on pharma companies [should NICE issue a negative decision], especially as rare diseases are often chronic compared to CDF- approved products, which often extend life in those with relatively low life expectancy. - ABPI-BIA Rare Disease Member Survey respondent
Adoption and implementation – embedding access and uptake
The final phase of the access journey determines whether innovative medicines actually reach patients in practice.
Even when rare disease treatments secure regulatory approval and reimbursement, uptake within the NHS can remain slow and inconsistent. This reflects longstanding structural barriers, complex commissioning arrangements, variable clinical readiness and an absence of clear, system-wide accountability for delivery.
Unlike other major conditions, there is no national framework or coordinated network for rare diseases. Expertise is dispersed, data is fragmented, and adoption often depends on local initiative rather than national design. This is compounded by a lack of data transparency on treatment adoption and patient outcomes via existing processes and services, creating uncertainty over the consistency of delivery of national standards.
This fragmentation also limits the NHS’s ability to generate consistent real-world data to inform future access decisions.
A clearer, connected national infrastructure is needed to link care delivery, data transparency, evidence generation, and service planning so that innovation reliably translates into patient benefit. The following proposals outline how this can be achieved.
The proposals that follow – appointing a National Clinical Director for Rare Diseases and establishing a network of NHS rare disease hubs – set out how stronger leadership, clearer coordination and better evidence infrastructure can ensure that new treatments are implemented consistently and equitably. Together, these reforms would help ensure that when innovative medicines reach the NHS, they also reach the patients who need them.
5. Appoint a National Clinical Director for rare diseases
Despite national commitments on rare diseases, leadership within the health system remains diffuse. Responsibilities for diagnosis, specialised services, genomics, workforce and digital infrastructure sit across multiple directorates, and no single clinical figure is accountable for bringing these elements together. This is in stark contrast to areas such as cancer, cardiovascular disease and mental health, which all benefit from named National Clinical Directors (NCDs) who translate strategic ambition into operational delivery and system change.
As a result, progress in expediting patient access to innovative rare disease medicines often relies on individual programmes or local initiatives rather than consistent national coordination.
A National Clinical Director (NCD) for Rare Diseases would address this gap. Appointed by DHSC and embedded within NHS England, the NCD would provide visible clinical leadership, align priorities across the system and help ensure that the NHS is prepared to adopt new therapies as they emerge. This role would connect specialised commissioning, genomics, pathway reform and data infrastructure, offering a focal point for improving consistency, reducing regional variation and sustaining momentum across the rare disease agenda.
Crucially, the NCD would provide a clear line of accountability for turning policy commitments into operational delivery and measurable improvement for patients.
6. Establish NHS rare disease hubs
For too many families, the rare disease journey is defined by fragmented care, delayed diagnosis and limited access to specialist expertise.
The challenge is not a lack of capability within the NHS, but rather that expertise remains dispersed, and the infrastructure to connect it is underdeveloped and under-resourced. As LifeArc and others have shown, this fragmentation undermines patient care, slows access to innovative therapies and limits the UK’s ability to generate the high-quality evidence that underpins modern appraisal and commissioning.
This is especially pertinent in the context of current NHS structural system changes as part of NHS England’s absorption into DHSC, which will have implications on the evolution of commissioning specialised services for patients with rare and complex conditions.
A structured network of NHS rare disease hubs would provide the clarity and consistency the system currently lacks. These hubs would bring together concentrated specialist expertise, diagnostics, genomics and care coordination, while maintaining strong links with local services through virtual Multi-Disciplinary Teams (MDTs) and shared records. The aim is not to centralise all provision but to ensure that every patient, regardless of geography, is connected to the expertise and specific clinical insight they need. The hubs would provide a clear, well-resourced clinical leadership infrastructure, supported by disease-specific leaders to guide the ongoing development of specialised services.
Such a model would also strengthen the UK’s evidence infrastructure. Consistent, high-quality real-world data – captured through shared standards and embedded digital tools – would support managed access schemes, inform commissioning decisions and underpin continuous service improvement. By joining up clinical care, research and data, hubs would help transform today’s uneven landscape into a coherent national system.
Structural enablers – establishing the framework for sustained reform
Effective reform of the rare disease treatment pathway requires more than process improvement; it depends on visible leadership to drive coherence, accountability and follow-through across the system.
Today, responsibility for rare disease innovations is currently dispersed between many different bodies across government, including the MHRA, NICE, NHS England, the Office for Life Sciences and the Department of Health and Social Care. Each has an important role, but the absence of a single coordinating mechanism means that promising policy developments often lose traction during implementation.
Strengthening structural leadership and modernising the service model are therefore essential. The UK needs clearer accountability at the centre, a refreshed national strategy that reflects today’s scientific and health-system context, and a contemporary service framework capable of supporting consistent, equitable delivery.
7. Appoint a National Rare Disease Champion
Over 3.5 million people in the UK live with a rare disease, yet these conditions continue to lack a visible national figurehead. Appointing a UK Government Champion for Rare Diseases would provide that leadership, acting as a cross-cutting advocate across health, research, and innovation policy.
The Champion should sit within the Office for Life Sciences – the joint-departmental unit spanning the Department of Health and Social Care, the Department for Business and Trade and the Department for Science, Innovation and Technology. Their mandate would be to align the delivery of the UK Rare Diseases Framework, coordinate across NHS England, NICE, and the MHRA, and ensure progress is tracked and reported publicly.
Crucially, the Champion would give rare diseases the same strategic visibility as other national health priorities, helping sustain political focus and drive accountability across government departments and agencies, while serving as a single, authoritative point of contact for patients, clinicians, and industry.
The Champion’s remit would also complement that of the proposed NCD for Rare Diseases. While the NCD would lead operational delivery within the health system – translating strategy into improved services and outcomes – the Rare Disease Champion would ensure cross-government alignment, policy coherence, and public accountability.
Together, these two distinct yet collectively reinforcing roles would help create a joined-up leadership model spanning government and the NHS, with one role driving system change from within, and the other championing rare diseases across departments and the wider life-sciences ecosystem.
8. Set a long-term vision for the next UK Rare Disease Framework
The UK Rare Diseases Framework has helped guide, shape and coordinate action across the four nations of the UK since its creation in 2021.
With the recent UK Government announcement to extend the current framework until January 2027, recognising the continued relevance of its priorities, the UK now has crucial opportunity to consider what should follow the current Framework and how best to embed rare diseases within a renewed, strategically aligned approach across the health and life sciences system.
Advances in genomics, cell and gene therapies, and advanced manufacturing are transforming what is possible for people living with rare diseases, while national strategies such as the 10-YHP and the LSSP reframe the UK Government’s broader ambitions for better health outcomes and globally competitive life sciences ecosystem.
A renewed Framework should build on the momentum these refreshed strategies offer.
It should bring together the ambitions of government, the NHS, regulators, industry, and the research community in a single, coherent plan; from discovery through to diagnosis, access, and long-term care. It should incorporate the practical solutions proposed in this report, ensuring that rare diseases remain a visible priority within the UK’s wider health and growth agenda. And it should place people with lived experience at its heart, ensuring that patients, carers, and advocates are active partners in shaping priorities, tracking delivery, and defining success.
Done well, a refreshed Framework would provide the leadership, accountability, and alignment needed to make the UK the best place in the world to research, develop, and deliver treatments for rare diseases.
9. Develop a new Modern Service Framework for Rare Diseases in England
The 10-YHP commits to new Modern Service Frameworks (MSFs) to define how the NHS delivers care for major conditions. Early priorities include cardiovascular disease, mental health and dementia. Crucially, these frameworks aim to set clear national standards, integrate pathways end-to-end, and embed digital tools and data into routine practice.
Rare diseases – which span multiple specialities, require coordinated care and depend heavily on timely diagnosis and specialist expertise – also have a strong rationale for inclusion within this initiative.
A MSF for rare diseases would formalise a single, end-to-end model of care – linking identification and diagnosis to treatment and follow-up – and embed the rare disease hubs within a nationally agreed pathway. By setting clear expectations and connecting services through shared digital infrastructure and data standards, an MSF would help ensure that innovation is adopted predictably and fairly across the NHS, regardless of geography.
By formalising the proposed network of rare disease hubs and embedding them within a nationally agreed model of care, an MSF would help ensure that no matter where a person lives, they can access the same level of specialist support and benefit from the same opportunities for early diagnosis and treatment.
Conclusion
A new way forward – from innovation to impact
The solutions detailed in this report represent a new way forward for improving access to rare disease medicines in the UK. Together, they offer a more coherent and predictable system that connects every stage of the access journey – from early regulatory engagement and appraisal through to commissioning, adoption and real-world evidence generation.
To make this happen, all parts of the UK life sciences ecosystem will need to work even better together.
This includes the MHRA and NICE continuing and intensifying existing efforts to create structured, joint early engagement as standard practice to ensure that evidence expectations are clear and proportionate from the outset. DHSC and NHS England should establish a dedicated, value-based pathway for rare disease medicines where traditional appraisals are not appropriate, drawing on the underused Innovative Medicines Fund to support earlier, evidence-linked access. NICE should continue to evolve its methods so that they recognise the long-term and societal value of innovation in rare diseases. And the NHS must ensure that implementation keeps pace with innovation.
Visible leadership will be essential to make this a reality. A UK Government Rare Disease Champion, working alongside a National Clinical Director and under a refreshed UK Rare Diseases Framework, would provide the strategic coordination, accountability and momentum needed to deliver lasting change. Industry, government, the NHS and patient organisations must work together to build a system that manages uncertainty pragmatically while ensuring patients benefit without unnecessary delay.
If implemented in full, these reforms would create a fairer, faster and more connected access pathway for rare disease treatments. Patients would gain earlier access to life-changing treatments; the NHS would benefit from a more efficient, evidence-driven allocation of resources; and the UK would strengthen its position as a global leader in rare disease innovation – turning scientific excellence into tangible outcomes for patients, the health service and the wider economy.
By acting now to modernise and better connect the rare disease access pathway, the UK can demonstrate how a coordinated, evidence-led system can deliver for patients, strengthen the NHS, and secure the country’s position at the forefront of health innovation.



