Forewords

Dr Danuta Jeziorska
Scientific founder and former CEO, Nucleome Therapeutics
Vice Chair of Data, AI and Genomics Committee (DAGAC), and Chair of Functional Genomics Subcommittee, BIA
The completion of the Human Genome Project marked a monumental achievement that transformed science and medicine. It offered, for the first time, a map of our genetic code.
But a map is not a manual, and our journey to truly unlock the power of the genome is far from over. We now stand at a pivotal juncture where our ability to read the genome must be matched by our capacity to understand how it works – to decipher the intricate mechanisms by which genomes dictate our health, how its disruption causes disease, and how it shapes the very nature of life itself.
This report outlines why functional genomics – the science of decoding the genome's operational instructions – represents a strategic imperative for the UK. By building upon our existing strengths and fostering a coordinated national effort, we can unlock unprecedented value across healthcare, agriculture, and environmental sustainability. I believe that by embracing functional genomics, the UK can not only solidify its position as a global leader in genomic innovation but also deliver tangible benefits to society for generations to come.

Dr Emma Lawrence
Head of Data Tech Policy and Public Affairs, BIA
The UK's leadership in genomics has positioned us uniquely to tackle the next major challenge in life sciences: deciphering the functional language of the genome.
This report from the BIA delves into the strategic importance of functional genomics for our sector, highlighting its potential to drive innovation across diverse applications, particularly in healthcare. By focusing on how genetic variations translate into biological impact, we can unlock new avenues for diagnosis, treatment, and the development of novel therapeutics.
The BIA is committed to supporting the growth of this dynamic field and believes that a cohesive national strategy is crucial to realising its transformative power for the UK's biotech ecosystem and the patients we serve.
Introduction
Understanding the genome's function is the critical next step in realising the full potential of life sciences. Functional genomics is the key to unlocking this understanding, decoding how genetic changes influence biological outcomes across all forms of life. The UK has a unique opportunity to leverage its existing genomics strengths to become a global hub for functional genomics-driven innovation, particularly in healthcare. This report highlights the transformative potential of functional genomics and the strategic importance of a coordinated national approach to capitalise on this pivotal moment.
What is functional genomics?
Functional genomics bridges the gap between our genetic code (genotype) and our observable traits and health (phenotype). This approach will help us better understand the mechanisms of disease, how genetic alterations impact health and target the right drug to the right patient.
What makes up a genome?
Our genome contains over 3 billion DNA letters, but only a small fraction (about 2%) are protein-coding regions of genes, which provide instructions for making proteins. The remaining 98%, once dismissed as "junk" DNA but now known as the "dark genome" or "non-coding genome," is crucial for controlling how and when genes are active. This "dark genome" acts like a complex set of switches and dials, directing our 20,000-25,000 genes to work together in specific ways, allowing different cell types (like nerve and muscle cells) to develop and respond to changes in the body. Essentially, while genes provide the basic instructions, the "dark genome" orchestrates their use.
From genes to the dark genome: how genetic changes disrupt health
While comparing DNA has revealed thousands of disease-associated genetic changes, understanding how these changes cause disease is complex. Unlike single-gene disorders, the vast majority of conditions, like chronic diseases are influenced by multiple—sometimes even hundreds—of genetic changes. This adds complexity to our understanding of how these changes lead to disease. To further complicate matters, only 10% of disease-associated genetic changes occur within genes. The remaining 90% are in the "dark genome". These changes can impact when, where, and how much of a protein is produced from a given gene, disrupting normal physiology.
Thousands of disease linked genetic changes for almost all common diseases identified.
While we have made significant progress in understanding how genetic variants affect protein function, major challenges remain in deciphering the full impact of these changes on biological processes. The complexity deepens even further when considering the dark genome, where most disease-associated variants reside. This is why functional genomics is critical. By combining genome sequencing with functional approaches, we can more accurately understand and therefore prevent or treat disease.
Functional studies enable precision medicine for breast cancer.
In certain cases of breast cancer, the HER2 gene becomes overactive, driving uncontrolled cell growth. Functional studies uncovered this mechanism, paving the way for diagnostics and targeted treatments like trastuzumab that directly inhibits HER2 activity. This highlights how understanding the relationship between genotype and phenotype enables precision medicine, where genetic diagnosis matched with tailored treatments significantly improves patients’ outcomes.

Rich Scott
CEO, Genomics England
Genomics is already changing lives through its impact in diagnostic use in cancer and rare disease. With continued investment in research and development, it could play a role in up to half of all healthcare encounters by 2035 as part of an increasingly prevention-focused health system harnessing increasingly tailored therapies.
Functional genomics will play an important part in this - building our knowledge of how genetic variation leads to cellular and clinical impact. The UK is uniquely placed to lead here - to be the place to discover, test and benefit from genomic innovations.
The transformative impact of functional genomics on the life sciences industry
Functional genomics is reshaping the life sciences, with a profound and far-reaching impact across healthcare and beyond. It enables a better understanding of disease risk, the discovery of biomarkers for earlier and more accurate diagnoses, the identification of novel drug targets and the development of personalised therapies that improve patient outcomes. Companies across the sector are leveraging functional genomics to refine disease models, optimise precision medicine strategies, and drive advances in synthetic biology. Beyond healthcare, functional genomics is also accelerating innovation in agriculture and environmental sustainability — supporting the development of climate-resilient crops, sustainable biomanufacturing processes, and next-generation bio-based materials. These examples highlight just a fraction of the field's broad potential to revolutionise industries that depend on biological systems.
Translational potential of functional genomics
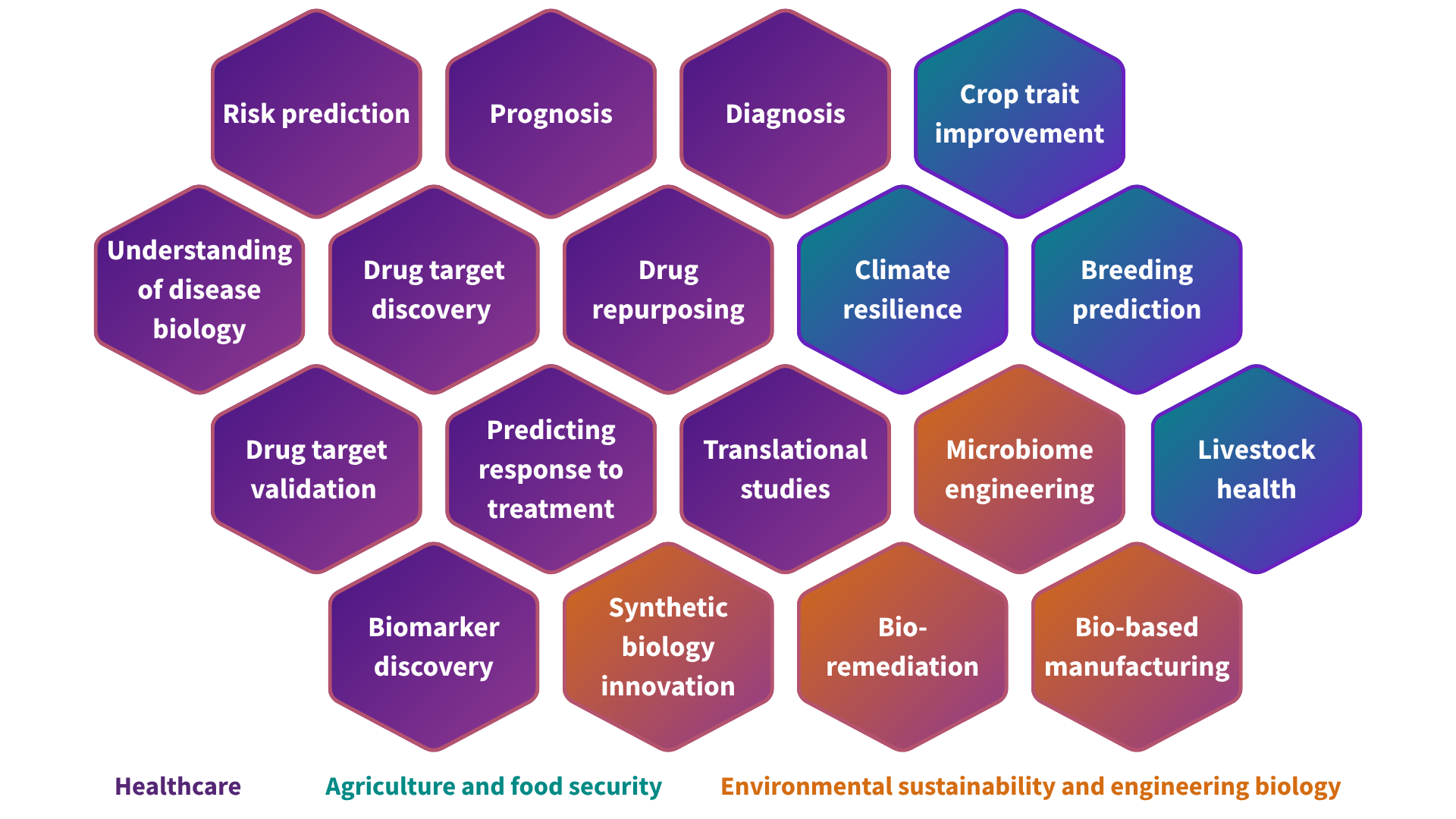
The UK is home to a world-leading commercial functional genomics sector, encompassing both innovative SMEs and large pharma companies. In the biotech industry, functional genomics is transforming the drug discovery pipeline. By linking genetic variations to disease mechanisms, companies can de-risk target discovery and improve drug development outcomes. Drugs based on genetic evidence are twice as likely to achieve market approval—a vital improvement in a sector where nearly 90% of drug candidates fail, with average development costs exceeding $1 billion and timelines spanning 10–15 years. UK-based biotech pioneers are at the forefront of this shift, using functional genomics to identify and validate existing and new drug targets, including those previously hard to target.
Diagnostic companies are harnessing functional genomics to develop next-generation tests that provide deeper insights into disease progression and treatment response. These technologies will also enable better prediction and prevention of disease.
Pharmaceutical companies have made significant investments in UK functional genomics initiatives, recognising the critical role of genetics in driving drug discovery and development. These investments span in-house capability building, strategic partnerships with UK-based biotech firms, and financial backing through their venture arms.

Nerida Scott
Regional Head of J&J external innovation group, EMEA
At Johnson & Johnson we believe that genetic and functional genomics initiatives will be transformative in helping us to enhance our understanding of disease, as well as the human relevance of R&D target selection, validation, and clinical trial success.
We have long-standing external collaborations and initiatives in the UK, such as UK Biobank (including The Pharma Proteomics Project), Our Future Health and many others that contribute world-leading data sets to this field.
Expertise in data science is a vital component in our internal R&D efforts, leveraging the power of artificial intelligence (AI) and machine learning (ML) and, as we seek the most promising early innovation from across the region, it will be a key consideration in our assessment of investments and collaborations. This is just the beginning.
For instance, in 2024 GSK partnered with Relation Therapeutics in a collaboration worth up to $300 million, which included equity investment. Nucleome Therapeutics has a partnership with Johnson & Johnson and has secured funding from the investment arms of three major pharmaceutical companies—Merck Serono, Johnson & Johnson, and Pfizer, further highlighting the sector’s growing appeal and recognised potential.
Beyond therapeutics, functional genomics is unlocking opportunities in engineering biology, where companies are utilising functional genomics data to enhance metabolic pathways and optimise bio-based production systems. Additionally, agricultural biotechnology firms are applying functional genomics to improve crop resilience, enhance disease resistance, and optimise livestock breeding, as explored further in our Deep Biotech report.
Spotlight: CardiaTec Biosciences
Spotlight: Constructive Bio
Spotlight: Entelo Bio
Spotlight: Milner Therapeutics Institute
Spotlight: Nucleome Therapeutics
Spotlight: PrecisionLife
Spotlight: Quotient Therapeutics
Spotlight: Relation Therapeutics
These examples illustrate just a fraction of the transformative power of functional genomics and its pivotal role in shaping the future of the UK life sciences sector. Realising its full potential requires a coordinated effort to integrate functional genomics insights across industries, ensuring that the UK remains at the forefront of this rapidly evolving field.
Why the UK
A solid foundation of leadership in functional genomics
UK academic excellence in functional genomics
The UK's strong foundation in genomics, evidenced by its academic excellence, collaborations, and key projects like Genomics England, has made it a global leader. These initiatives have provided crucial data. The next step is to focus on functional genomics to understand the meaning of this genetic information. Although functional research exists, a coordinated national initiative is essential to move beyond sequencing and fully realise the potential for future innovation and impact.
Integrating technologies to uncover disease mechanisms in the genome
Identifying disease-driving variants and understanding their effects requires an integrated functional genomics approach. Functional genomics combines multiple cutting-edge techniques, each providing unique insights into how genetic changes impact cell function and cause disease. Just like needing all the pieces of a puzzle to see the full picture, only by integrating these diverse data can we move beyond correlative links to causal insights. As each cell type (e.g., nerve, skin, muscle) has distinct patterns of gene activity, it’s essential to study these changes in specific cell types rather than in mixed populations of cells (e.g., red and white cells in blood), which might mask crucial details.
AI and data integration play a crucial role in functional genomics, as it generates enormous datasets from diverse methods, each capturing different layers of cell biology. Machine learning and advanced data integration allow researchers to extract insights from this complexity, identifying patterns and predicting variant effects on health.
Translational models: more accurate cellular models that replicate the complexity of human diseases, bridging the gap between preclinical studies and real-world applications are urgently needed.
AI and data integration - Translational models
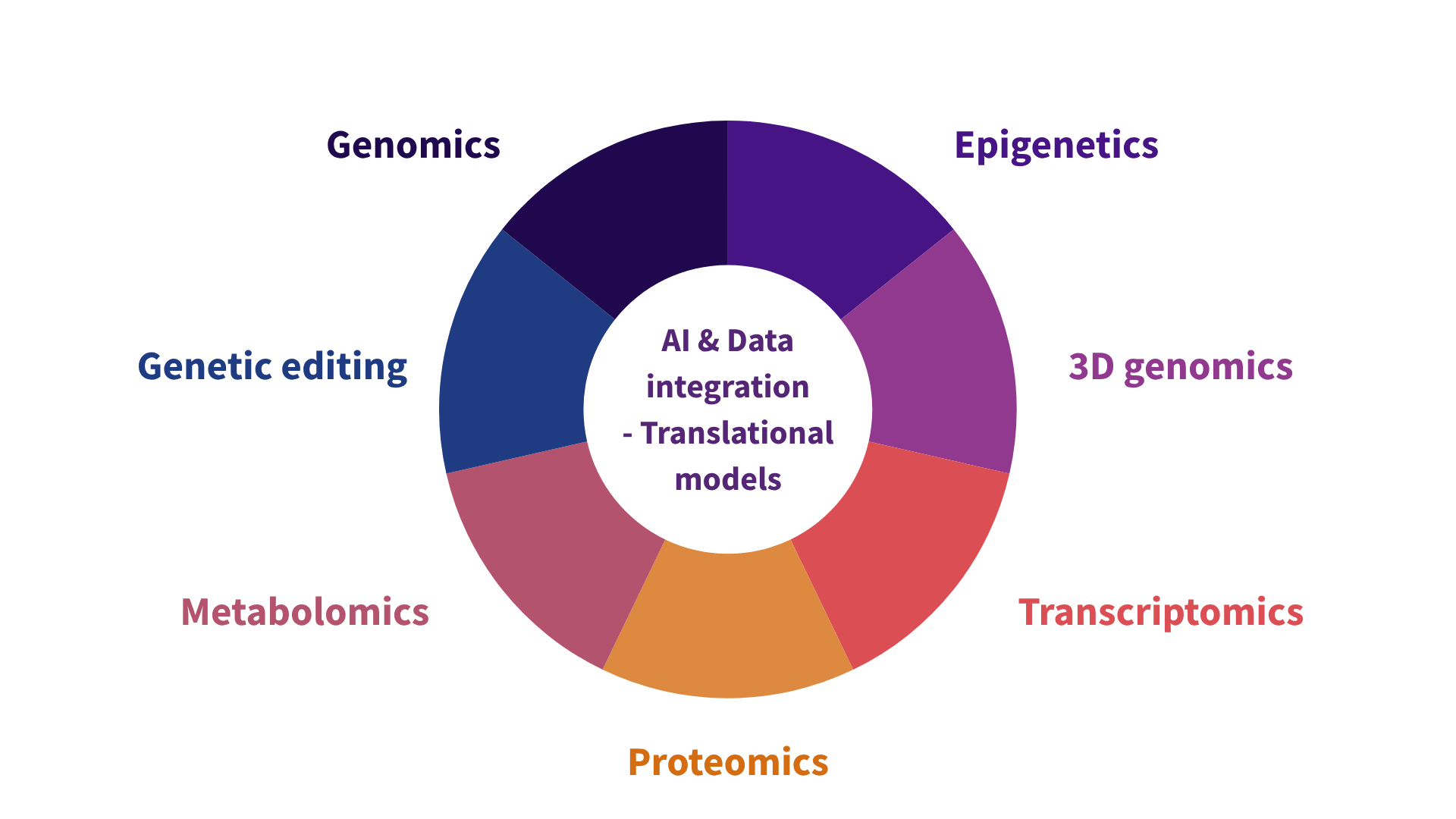
Genomics: identifying genetic variants
By mapping genome sequence genetic variants associated with diseases can be detected, whether in genes or the dark genome. Identifying variants is only the starting point; understanding their impact requires additional approaches.
Epigenetics: mapping and classifying regulatory elements
By mapping location of regulatory elements across cell types and classifying them based on functional activity, we can define if variants interfere with gene regulation, leading to diseases.
3D Genomics: mapping variants to impacted genes
By mapping how the genome folds in the cell nucleus, distant regulatory elements in the dark genome can be linked experimentally to genes they regulate. This makes it possible to connect dark genome variants to gene expression, revealing their functional role and mechanism in disease.
Transcriptomics: measuring gene activity
Measuring RNA levels provides a snapshot of which genes are actively producing transcripts in specific cell types and conditions. This is essential for identifying impact of genetic changes on genes function in disease.
Proteomics: observing functional outputs
Proteomics measures the levels and activities of proteins, which are the final functional products of genes. Changes in protein levels or activities provide direct evidence of how genetic changes disrupt cellular function.
Metabolomics: analysing cellular metabolism
Metabolomics tracks small molecules in cells, offering insights into changes in metabolic pathways influenced by genetic variants.
Gene editing: confirming causal impact
Methods like CRISPR/Cas9 enables precise manipulation of specific genetic sequences in genes or the dark genome. By introducing targeted changes, scientists can observe the effects on gene expression and cellular behaviour. The dark genome’s genetic variants needs to be first precisely linked to genes they impact
Spotlight: the UK’s academic excellence in functional genomics
The UK’s genomics strength is underpinned by its robust academic excellence distributed richly across the country. From the Wellcome Genome Campus, home to the Sanger Institute, to university-led breakthroughs, this fundamental research plays an important role in advancing knowledge of how the genome functions. These institutions are also driving the discovery of innovative tools and methodologies to decode the genetic changes in both coding and dark genome regions.

Professor Matthew Hurles
Director and Senior Group Leader, Wellcome Sanger Institute
At the Sanger Institute, we see first-hand how large-scale functional genomic studies are crucial for connecting genetic information to biological impact to tangible health outcomes. This work is vital for translating genomic discoveries into real-world impact for patients.
The UK’s response to the COVID-19 pandemic demonstrated its ability to mobilise functional genomics expertise rapidly across sector, producing life-saving insights. The UK has also fostered several collaborative initiatives that bring together academia and industry, including Open targets, Human Cell Atlas and Atlas of Variant Effect.
- Functional genomics reveals life-saving insights into COVID-19 risk
Researchers at the University of Oxford discovered that a genetic variant doubling the risk of severe COVID-19 was located in the dark genome, affecting lung cell function rather than the immune system. This discovery underscored the importance of vaccination strategies for at-risk populations.
- Open Targets
Open Targets is a pre-competitive partnership that combines expertise from academia and the pharmaceutical industry. It supports the systematic identification and prioritisation of drug targets using publicly available human genetics and functional data. It brings together world-class research institutions, including the European Bioinformatics Institute (EMBL-EBI), the Wellcome Sanger Institute, and five leading pharmaceutical companies. The consortium was established 10 years ago to capitalise on UK-based genomics and functional genomics expertise to develop an open-source data and informatics platform: Open Targets Platform. It has funded over 90 projects so far, uncovering novel targets and, generating and sharing foundational datasets for drug discovery research with over 70,000 registered users. However, the platform relies heavily on existing datasets, meaning it is limited in data completeness and the mapping of dark genome variants to function is imprecise remaining a challenge.
- Human Protein Atlas
The UK is one of the major data contributors into the Human Protein Atlas. This is a Swedish-led initiative that maps the expression and localisation of all human proteins across different tissues and cell types. By integrating data from transcriptomics, proteomics, and imaging technologies, it provides a detailed view of protein function in health and disease. This resource is widely used by researchers and drug developers to identify disease biomarkers, validate drug targets, and explore protein functions at a system-wide level.
- Atlas of Variant Effects
The Atlas of Variant Effects is an international initiative that aims to systematically characterise the functional impact of genetic variants at scale. By combining experimental and computational approaches, it seeks to develop a comprehensive map of how different genetic changes influence gene function and contribute to disease. This resource is particularly valuable for improving variant interpretation in clinical genomics and enhancing precision medicine efforts. The UK, with its strong genomics research infrastructure, is a key contributor to the initiative, supporting efforts to generate large-scale functional datasets and integrate them into biomedical research and clinical practice. Much of the functional data generated to date focuses on protein-coding regions, leaving significant gaps in the understanding of how regulatory variants in the dark genome contribute to disease.
This culture of collaboration, coupled with scientific excellence, serves as a catalyst for breakthrough innovations, fostering the emergence of world-leading companies and nurturing a talent pipeline critical for the growth of the functional genomics sector.
- Human Cell Atlas
The Human Cell Atlas (HCA) is an international collaborative consortium launched in 2016 in the UK with the mission of creating comprehensive reference maps of all human cells, the fundamental units of life. By integrating cutting-edge single-cell transcriptomics and spatial genomics with advanced computing and AI methods, the HCA community is generating high-quality atlases of tissues, organs, and systems. The initiative was co-founded by scientists from the Wellcome Sanger Institute and the Broad Institute and has major contributions from UK-based researchers. Its datasets are already transforming understanding of tissue organisation, immune responses, and disease mechanisms, laying critical foundations for functional genomics and precision medicine. Atlases of the lung, brain, retina and organoids are already available through the HCA Data Portal, with additional organ-level datasets to follow in the next years, then expanding towards the ultimate goal of a complete, three-dimensional, and globally representative Atlas of the human body.
Professor Sarah Teichmann, co-founder and co-Chair of the HCA, from the University of Cambridge, said: “If the Human Genome Project gave us the 'book of life', the Human Cell Atlas captures which chapters of the book are read to make each cell of the body. This is a hugely exciting time for functional genomics, with HCA discoveries already informing medical applications from diagnoses to drug discovery. The Atlas will impact every aspect of biology and healthcare to benefit humanity across the world.”
Strategic investments in functional genomics
Laying the foundations
Recent strategic investments lay the foundation for consolidating the UK’s leadership in functional genomics. In 2023, the UK made substantial investments to advance functional genomics capabilities through advanced infrastructure, talent development, and cutting-edge research. The Medical Research Council (MRC), in collaboration with the Biotechnology and Biological Sciences Research Council (BBSRC) and other stakeholders, committed £28.5 million to launch the Human Functional Genomics Initiative, which included establishment of four CRISPR screening laboratories. One of these labs, at the Milner Therapeutics Institute, was set up in collaboration with AstraZeneca and the University of Cambridge.
AstraZeneca has also extended its partnership with Cancer Research Horizons (CRUK) on a joint AZ-CRH Functional Genomics Centre to discover novel mechanisms of sensitisation and resistance to oncology drugs. Having supported nearly 100 projects, the partnership was renewed for another five years in 2023, marking the first major biopharmaceutical investment in UK functional genomics in collaboration with a charity.

Steve Rees
SVP, Discovery Sciences, R&D, AstraZeneca
Genomics, particularly functional genomics, plays a pivotal role in advancing pharmaceutical R&D by uncovering new drug targets, biomarkers, and mechanisms critical to drug discovery.
The partnerships we have in the UK, such as the Cancer Research Horizons-AstraZeneca Functional Genomics Centre and the Medical Research Council (MRC)-AstraZeneca-University of Cambridge Functional Genomics Screening Laboratory (FGSL) at the Milner Therapeutics Institute, demonstrate the breadth of genomic capabilities and how we are working together to support the development of life-changing medicines.
To accelerate progress, we continue to invest in innovation and collaborations that unite expertise and resources across the globe, ensuring that discoveries are inclusive, impactful, and tailored to address diverse healthcare needs of communities worldwide.
Additionally, £46 million was awarded to the University of Edinburgh in 2024 to develop advanced infrastructure, attract world-class talent, and deepen our understanding of genomic regions critical to health and disease.

Professor Wendy Bickmore
Director: MRC Human Genetics Unit, Institute of Genetics and Cancer, University of Edinburgh
We hear so much about the human genome that you’d be forgiven for thinking it’s a code we’ve cracked and we can now move on and reap the rewards. But in fact our knowledge of the human genome in its entirety barely scrapes the surface.
That’s because our genes make up < 5% of the human genome - the rest - sometimes referred to as “dark matter” we have little understanding of. Yet, it’s this part of the genome that contains the information that controls our genes - switching them on and off at the right times and in the right cells and tissues. There are hundreds of thousands of these switches (enhancers) and we know changes in their DNA sequence can influence our risk of developing disease.
Functional genomics research in the non-coding genome is difficult because enhancers function in a every cell and tissue-specific context. Funding from the MRC to the Human Genetics Unit is enabling us to develop the methods needed to understand how changes in enhancers affect gene control in disease relevant complex biological systems, including organoids and animal models.
Together, these investments strengthen the position of the UK as a leader in functional genomics, accelerating innovation and translating discoveries into impactful healthcare solutions, laying the foundation for the sector.
Next steps for maintaining the UK’s genomics leadership
Functional genomics presents a pivotal opportunity for the UK to move beyond competing on the sheer number of genomes sequenced or the scale of its longitudinal datasets with other nations. The country is poised to pioneer the next frontier in genomics, focusing on the deep interpretation of genetic and functional data to deliver actionable insights that transform healthcare.
A nationally coordinated effort in functional genomics would not only enhance the impact of past genomic investments and solidify the UK’s position as the global hub for genomics-driven healthcare innovation. Such leadership would attract international partnerships and investment, reinforce competitiveness on the global stage, and accelerate the delivery of precision medicine to patients.
With the powerful tools and knowledge, the time is right for a bold move in functional genomics. These efforts will unlock the full value of the immense sequencing data already collected, transforming it into actionable insights for diagnosing, treating, and even preventing diseases. Creating AI-ready datasets at scale that link sequence to function is foundational and critical for building AI-driven predictive medicine, as well as critical to the development of engineering biology. Functional genomics is also central to the development of reliable pre-clinical models that more faithfully replicate human biology providing better prediction of the therapeutic outcomes. As we deepen our understanding of disease biology through the lens of genetics, we move closer to realising precision medicine—where therapies can be customised to restore normal cellular function in patients based on their unique genetic makeup, ensuring targeted and effective treatment.
To fully harness this potential and build on the UK’s global advantage, we should continue to invest in a nationally coordinated programme for functional genomics. This should include:
-
Coordinated investment to understand disease biology by decoding the causal link between genotype-phenotype. This should be done for both coding and non-coding genome (e.g. dark genome) variants in different disease areas and cell types (starting with a focused approach).
-
Collaborative infrastructure that enables the sharing of data, tools and techniques.
-
Enhanced data standards, data linkage and platforms for bringing together disparate genomics data.
-
Development of pre-clinical models which better represent human biology and are more reliably predictive of therapeutic outcomes in humans.