The hidden socioeconomic impacts of rare diseases in the UK

In this blog, Emily Klein, Senior Policy and Public Affairs Executive at BIA, explores the hidden, wide-ranging impacts and costs of rare diseases to society and the wider economy in the UK. It draws on insights from a pragmatic literature review by Costello Medical, commissioned by the BIA’s Rare Disease Industry Group (RDIG), a group of BIA member companies committed to improving patient access to rare disease treatments.
Rare conditions may be individually uncommon, but collectively they affect millions. Around 3.5 million people in the UK are living with a rare condition, many of which are severe, disabling and begin in childhood. Yet treatment options remain scarce, with only 5% of rare conditions having a licensed therapy, despite cutting-edge advances from the life sciences sector.
Living with a rare condition carries profound inequities and hidden social and economic costs for patients, families, carers, and society, including significant diagnostic delays, highly specialised, expensive medical and social care costs which often result in high productivity losses. These costs are hard to quantify given the heterogeneity of over 7,000 rare conditions spanning multiple specialties and the lack of robust, consistent data.
Currently, NICE’s medicine appraisal process only considers NHS and Personal Social Services costs, and patient and caregiver quality of life (QoL) in its cost-effectiveness analyses. For rare conditions, where evidence is inherently limited and uncertainty is high, it is often challenging for medicines to demonstrate value under current frameworks. Many therapies deliver benefits that extend beyond direct clinical outcomes, yet these benefits are not fully captured in NICE’s quality-adjusted life year (QALY) based assessments.
To understand the socioeconomic burden of rare diseases in the UK, the BIA commissioned Costello Medical to conduct a pragmatic literature review on the socioeconomic burden of rare diseases in the UK. The review explored direct and indirect costs[i] and qualitative insights describing patient and caregiver socioeconomic burden[ii], across a representative selection of ten rare and ultra-rare conditions[iii], using publicly available information from published NICE appraisals and literature relevant to the UK population where available.
What the review reveals
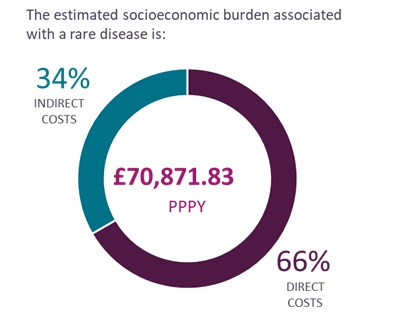
Based on the ten conditions explored, which span several therapeutic areas, the review estimates the socioeconomic burden associated with a rare condition in the UK is approximately £70,000 per patient per year (PPPY), made up of 66% direct costs and 34% indirect costs.
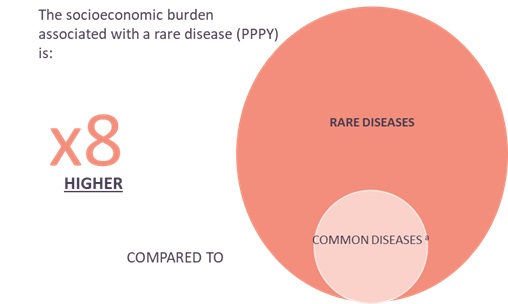
The socioeconomic burden associated with rare diseases in the UK is estimated to be approximately eight times higher compared to common conditions like diabetes, cardiovascular disease, cancer and Alzheimer’s. Assuming that a cost of £70,000 PPPY is representative of UK socioeconomic burden across all rare diseases, when applied to the estimated 3.5 million people living with a rare disease in the UK, this equates to an estimated annual cost of approximately £200 billion per year[iv].
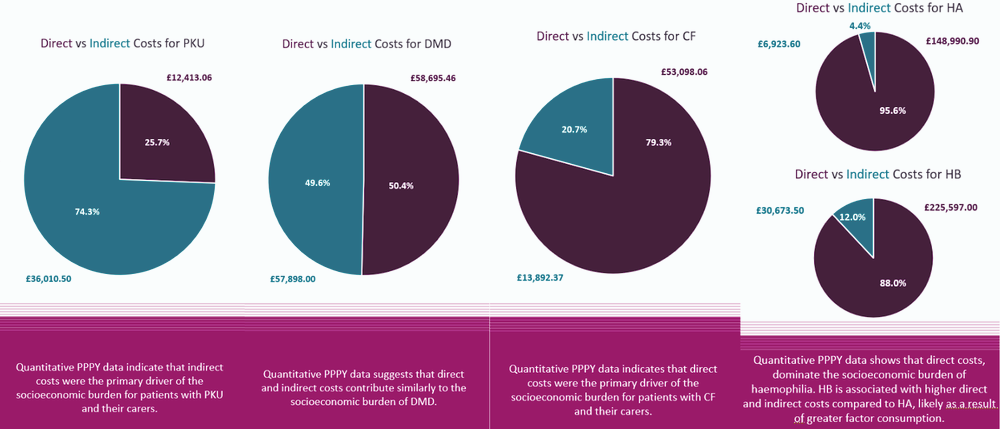
Burden was found to be predominantly driven by direct costs, with costs extending beyond direct medical expenses, while indirect costs represented a smaller share of the annual cost burden. However, indirect costs were generally found to be underreported in the literature and are therefore likely to be underestimated in this study. That said, for some conditions including Phenylketonuria (PKU) and Duchenne Muscular Dystrophy (DMD), indirect costs were a key driver of socioeconomic burden for patients and carers.
Key results include:
Qualitative insights highlight the wide-ranging impacts of rare diseases on individuals and families, with consistent themes including reduced productivity, limited career aspirations, reduced earnings, and negative impacts on schooling.
Quotes from the review include:
Patients frequently report having to reduce their working hours due to chronic AHP symptoms or give up work altogether. This reduction in work capacity is likely to lead to increased government expenditure on unemployment benefits and statutory sick pay and decreased government revenue from income tax and National Insurance contributions.[v]
Many DMD caregivers terminate their employment or reduce their working hours to find the time needed to care for their sons, and those who do continue to work have markedly impaired productivity with high levels of absenteeism […].[vi]
Together, these findings provide a clear picture of the scale and impact of rare diseases and confirm socioeconomic burden of rare diseases in the UK is very high. Treatments can help to alleviate this burden for patients and families and deliver societal benefits, demonstrating the need to consider both economic and social factors within value assessments and policy decisions.
The opportunities ahead
These insights come at a pivotal time. The UK’s commercial environment and policy landscape is evolving, with changes to NICE’s cost-effectiveness thresholds, an emerging new regulatory pathway for rare disease therapies, growing political discourse around the economic value of medical innovation and a review of the UK Rare Diseases Framework.
These shifts create an opportunity to translate ground-breaking innovation into positive outcomes for patients. The BIA’s new report, developed by the BIA’s Rare Disease Industry Group (RDIG), sets out solutions to unlock patient access to rare disease medicines that respond to the challenges highlighted in the literature review, including evolving NICE’s methodology to enable greater flexibility in measuring value to reflect the full spectrum of clinical, social and economic benefits that treatments can bring.
2026 is set to be a crucial year for shaping the future direction of rare disease policy in the UK. Expanding appraisal frameworks would be a vital step toward addressing long-standing barriers and accelerating access to transformative, potentially life-saving treatments for patients with high unmet need.
The BIA will continue driving our policy solutions forward in 2026 and beyond, ensuring developments in the medicines policy environment and ambitions for UK life sciences are aligned with the needs of rare disease patients.
[i] Direct socioeconomic costs and resource use, include but are not limited to medical expenses, healthcare service use and costs, travel and accommodation costs, childcare costs, private healthcare costs and formal/professional caregiver costs. Indirect costs include other categories of socioeconomic burden, that may not currently be considered (explicitly) within the existing NICE value framework but have significant impacts on UK society – including but not limited to loss of productivity due to illness or disability, absenteeism from work, reduced workforce participation and informal caregiver costs.
[ii] Patient and caregiver QoL include qualitative insights from various sources of literature
[iii] The 10 conditions included in the literature review were: Phenylketonuria (PKU), Cystic Fibrosis (CF), Acute Intermittent Porphyria (AIP), Haemophilia (A and B), Myasthenia Gravis (MG), Juvenile Idiopathic Arthritis (JIA), Bardet-Biedl Syndrome (BBS), Paediatric-Onset Hypophosphatasia (HPP), Metachromatic Leukodystrophy (MLD), Duchenne Muscular Dystrophy (DMD)
[iv] Please note the average annual cost (PPPY) across the 10 selected conditions has been translated to the number of individuals living with a rare disease in the UK. This extrapolation has its limitations as it assumes that the selected conditions are representative.
[v] 1. Kauppinen RK et al. Eur J Neurol. 2020;27(S1):1–102 (cited within Submission for NICE HST16 – Committee Papers, November 2021. Available at: https://www.nice.org.uk/guidance/hst16/evidence/evaluation-consultation-committee-papers-pdf-10888958269
[vi] Landfelt E et al. PharmacoEconomics. 2017;35:249–258






