Sustainable medicines manufacturing
Meeting sustainability goals without compromising product quality or safety
As the world becomes increasingly focused on environmental responsibility, the bioprocessing industry is transforming its approach to reduce its environmental footprint without compromising quality or safety.
Today’s medicine manufacturing involves a wide array of complex, resource-intensive processes, from chemical synthesis in traditional pharmaceutical production to biological methods in bioprocessing, which uses living cells to develop drugs and vaccines. Both sectors face unique challenges in reducing waste, conserving water and energy, and controlling emissions.
Latest case studies
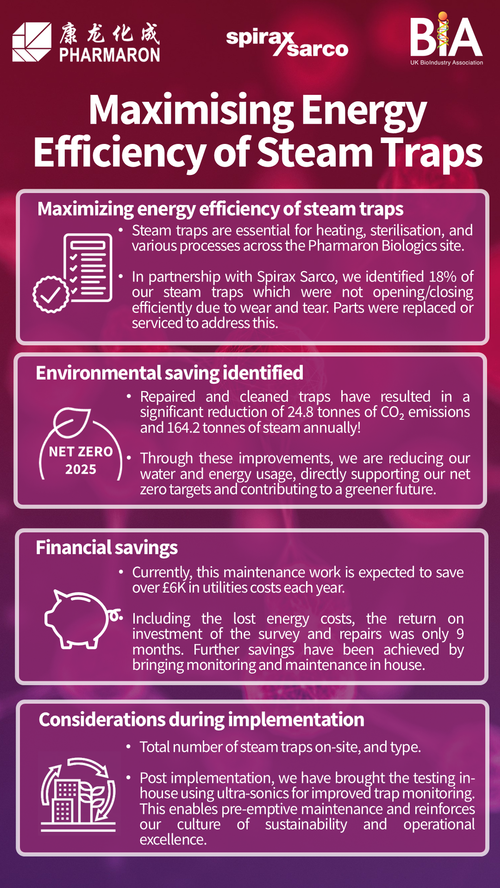
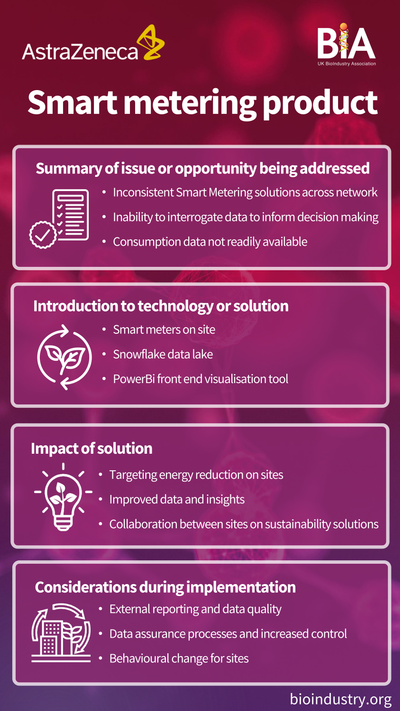
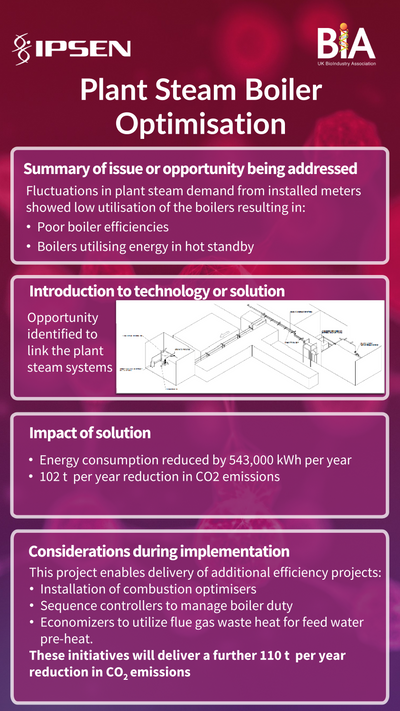
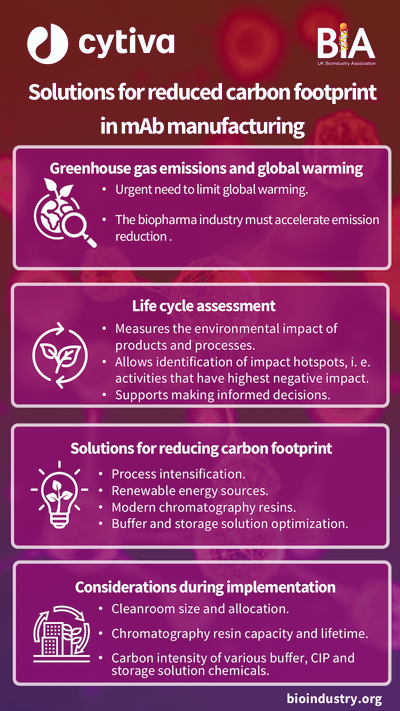
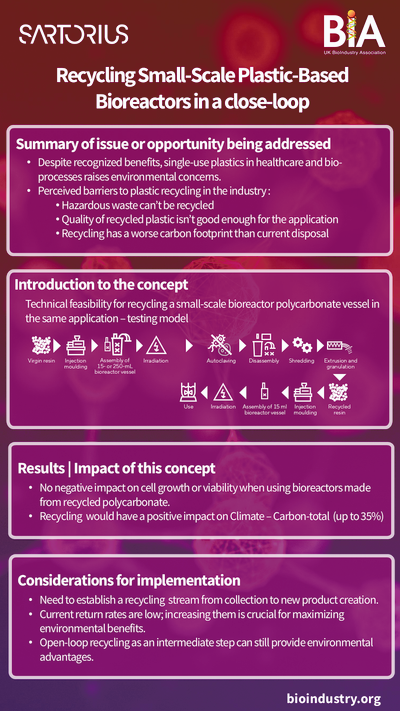
In the bioprocessing sector, where biologics, vaccines, and cell-based therapies are produced using live cells, sustainable manufacturing requires different approaches. Bioprocessing often involves significant use of single-use plastic systems for sterility, which can lead to high levels of plastic waste. However, the sector is innovating with new biodegradable and recyclable single-use technologies, allowing manufacturers to reduce waste without compromising product quality or safety.
Bioprocessing companies are implementing techniques such as process intensification, where processes are streamlined to use fewer materials, energy, and water. By optimising cell culture conditions and nutrient delivery, for instance, companies can achieve higher yields from smaller batches, leading to less resource consumption per unit of product.
Each case study featured here dives into real-world applications of sustainable practices. By rethinking every stage of production, from facility design, resource sourcing and process design to waste management, BIA’s MAC companies are creating sustainable models that can inspire similar changes across the industry.
The BIA MAC’s Sustainability working group recognises the need to collaborate to drive sustainability in biopharma manufacturing. In 2024, we set out an ambitious plan to create a place where companies can share their sustainability case studies in order to accelerate adoption of technologies and strategies for making medicines more sustainably. It is great to see this materialise, I hope that this facilitates the adoption of the technologies shared to help make our industry more sustainable.