Verona Pharma Announces Analyses Demonstrating Ensifentrine Reduced Exacerbation Rates Across Subgroups in Phase 3 ENHANCE-2 Trial for COPD
Verona Pharma plc (Nasdaq: VRNA) (“Verona Pharma” or the “Company”), announces additional exacerbation* subgroup analyses from the Phase 3 ENHANCE-2 (“Ensifentrine as a Novel inHAled Nebulized COPD thErapy”) trial in chronic obstructive pulmonary disease (“COPD”). Top-line results from the trial are available on Verona Pharma’s website here.
As previously reported, in the overall study population, ensifentrine demonstrated a 42% reduction in the rate of moderate to severe exacerbations over 24 weeks compared to those receiving placebo (p=0.0109). Results of the subgroup analyses confirmed positive effects consistent with the exacerbation reduction observed in the overall population across all subgroups analyzed over 24 weeks. ENHANCE-2 was not powered for exacerbation rate in subgroups.
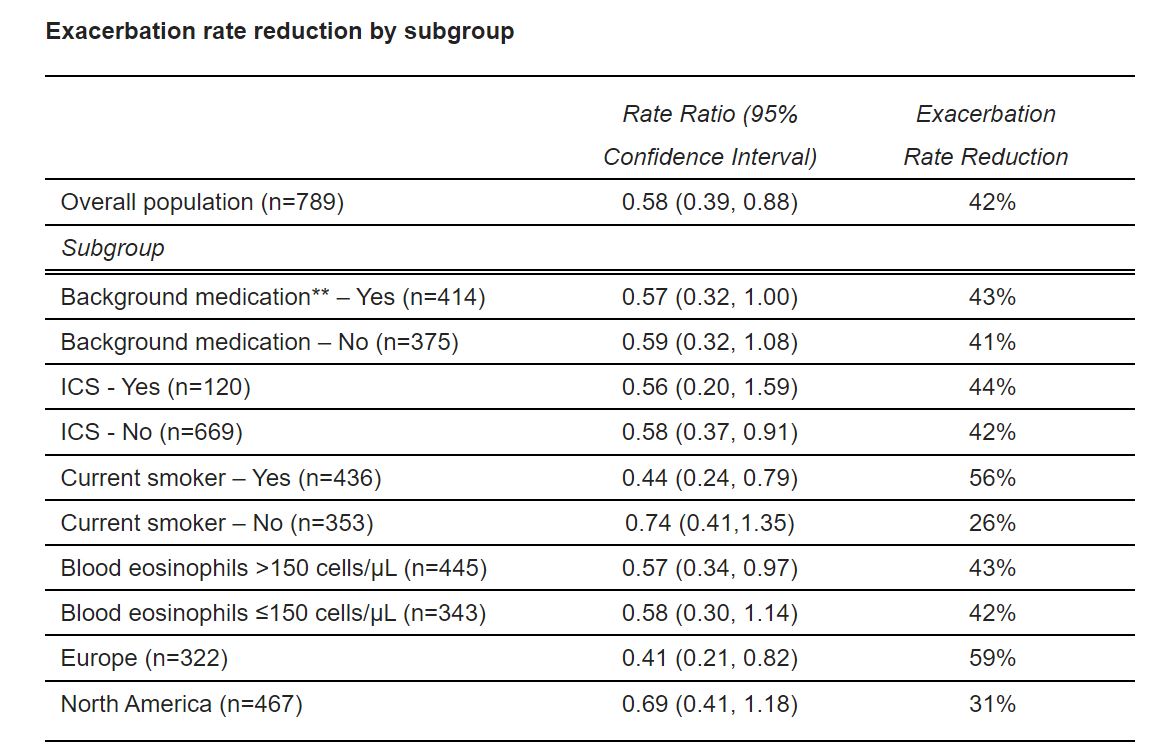
*An exacerbation was defined as a worsening of symptoms (two major or one major and one minor) requiring minimum of three days treatment with oral/systemic steroids and/or antibiotics or hospitalization
**Background medication included either a long-acting muscarinic antagonist (“LAMA”) or a long-acting beta-agonist (“LABA”). Approximately 15% of subjects also received inhaled corticosteroids (“ICS”)
“We are very encouraged by the meaningful and consistent reductions in rates of COPD exacerbations across all subgroups analyzed,” said David Zaccardelli, Pharm. D., President and Chief Executive Officer. “Despite treatment with available therapies, many COPD patients continue to experience exacerbations, which are estimated to cause approximately 1.9 million emergency department visits and 740,000 hospitalizations per year in the United States alone. Pending assessment of the results from our ongoing Phase 3 trial, ENHANCE-1, which is on track to read out around the end of 2022, these exacerbation data will be included in the New Drug Application in the US, which we expect to submit in the first half of 2023.”
Verona Pharma plans to release additional information from ENHANCE-2 at upcoming scientific conferences.
