Achieving equity for people living with rare diseases

This year, Rare Disease Day is being celebrated on 28 February, raising awareness and working towards equity in social opportunity, healthcare, and access to diagnosis and therapies for the 300 million people worldwide living with a rare disease.
In this blog, Senior Policy and Public Affairs Executive at BIA, Joe Smale, explains the difference between equality and equity and reflects on the findings of recent BIA research, indicating public support for achieving equity of access to treatments for people living with rare diseases.
In health systems designed for common diseases, people living with rare diseases face inequities in accessing diagnosis, care and treatments. This year for Rare Disease Day, BIA is joining the wider rare disease community to raise awareness of the need to achieve greater equity for people with rare diseases.
What’s the difference between equality and equity?
So, what is equity and how does it differ from the notion of equality? The below image does a good job of illustrating the difference.
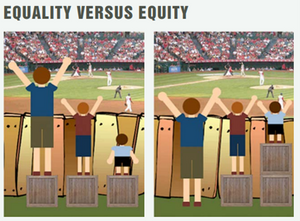
In the left picture, spectators of the baseball game are being treated equally as it is assumed that they will all benefit from the same support. In the right picture, however, the spectators are being treated equitably since they are given different support, according to their needs, to enable them to have equal access to the game.
Now imagine that the child in blue represents the 3.5 million people in the UK living with a rare disease. Ensuring that these people can receive access to diagnosis, care and treatment on an equitable basis to others in society requires the provision of additional support within the healthcare system to account for their needs.
Equity of access to treatment
Accessing treatments on an equitable basis is an aspiration that has not yet been realised for people living with rare diseases. An example of this reality can be seen in the time taken for a new treatment to become available to patients from the point of a treatment receiving marketing authorisation.
According to the EFPIA Patients W.A.I.T indicator, it took 98 days longer on average for non-oncology rare disease medicines to be made available to patients during 2017-2020 in England when compared to other innovative medicines. The data also shows that this delay is 22 days longer than the average delay in Europe. Whilst there is no single cause for these delays, eliminating them is an important piece of the puzzle in achieving equity of access to treatment for people with rare diseases.
Public support
BIA’s Rare Disease Industry Group (RDIG) recently commissioned a piece of research to explore the public’s opinions on how treatments for rare diseases are funded and how funding decisions are made. While the key findings of this research will be published in due course, some of the results are poignant to this discussion.
During the research, the BIA sought to understand the degree of public support for equity of access to treatment for people with rare diseases. In an online survey of a representative sample of 1,000 people, 80% of respondents agreed that people living with rare diseases should have equitable access to medicines, even if this means extra costs for the NHS. This suggests that the public would support measures to improve equitable access to medicines for people with rare diseases.
The findings of the research also showed that the majority of respondents (68%) agree with spending more money on helping someone with a rare disease than someone with a common disease. This is an encouraging indication that the public would support additional NHS measures to improve equity of access to rare disease treatments.
The route to equity
One route to delivering greater equity of access to people with rare diseases is through already established policy initiatives and frameworks.
On this year’s Rare Disease Day, the Department of Health and Social Care will publish the second instalment of the England Rare Disease Action Plan, setting out new actions for the implementation of the UK Rare Disease Framework in England. The plan will include 13 new actions, including 5 specifically dedicated to improving access to specialist care, treatment and drugs for people with rare diseases.
BIA welcomes the publication of the 2023 Action Plan and looks forward to working with its delivery partners to support the implementation of these new actions. In particular, BIA looks forward to sharing the findings of our recent research with individual delivery partners to support the case for implementing measures to enable people with rare diseases to access treatments on an equitable basis to others.
More like this
.png)
.png)