UK companies driving cell and gene therapy: Purespring case study

Purespring is revolutionising the treatment of kidney diseases with gene therapies that target a single kidney cell, the podocyte. The latest BIA's UK cell and gene therapy report explores the usage of Purespring's podocyte and the challenges and opportunities the company has had to navigate.
What does the company do?
Purespring Therapeutics is the first gene therapy company looking at the glomerulus in the kidney, which has thus far been under-examined as a target. The company is developing two programmes at present and is also exploring other targets that will allow the pipeline to be expanded in the future. Crucially, Purespring is built as a long-term, highly integrated company that will be able to take treatments not just to the clinic, but to market.
How will it be used?
Podocytes in the glomerulus are implicated in a large number of kidney diseases, and in many ways are ideal targets for gene therapies. They are not recycled, and do not divide, so poor function will persist throughout a patient’s life – conversely, however, this means that any gene therapy will also be persistent. Purespring’s platform has the potential for identifying and developing treatments for a large number of kidney diseases with unmet needs.
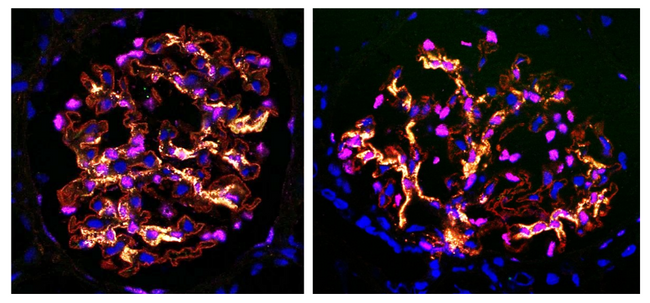
Prespring’s technology delivers transgenes selectively to podocytes with high efficiency.
Experiments have demonstrated that Purespring’s gene therapy expressed transgene protein (yellow) in kidney podocytes (red cytoplasm & membrane, pink nucleus) but not in other cells (blue nucleus).
What is the impact?
Initially, the focus will be on rarer monogenic nephrological conditions and the potential that exists for a fully curative treatment. Depending on the condition this can lengthen patient life, increase quality of life dramatically, or both. Other developments in kidney therapeutics recently have focused on managing symptoms, and haven’t directly targeted the disease pathway, so Purespring’s approach stands out.
What are the opportunities and challenges?
Opportunities: Purespring’s pipeline currently focuses on monogenic diseases, but their approach is not constrained to this. The ability to look at multigenic diseases, especially those beyond the rare disease space, is a major opportunity, especially when combined with Purespring’s established integrated capabilities.
Challenges: However, this is also a challenge – as an SME with fewer than 50 personnel, Purespring currently lacks the capacity to deliver all the programs that are possible with the platform. Finding further investment and expanding infrastructure will be needed before they can fully deliver on the promise.
From a technical perspective, the primary challenge in expanding to multigenic indications is to deliver treatments via the bloodstream while maintaining patient safety and avoiding off-target effects elsewhere in the body.
Why should investors invest in this space?
In addition to the broader developments in cell and gene therapies that are common to the space, this is an excellent time for nephrology. Purespring views it as a cresting wave, with a growing understanding of the pathology of kidney diseases and potential targets driving increased interest from investors and big pharma, and in turn, this is leading to more biotechs launching in the space. The end result is more success in the past five years than in decades of previous work.
What are the future trends for your products/processes?
With the increasing success of gene therapy projects and a growing list of approved treatments in various markets, Purespring expects an almost exponential wave of commercially available gene therapies. However, so far most of these have been for rare diseases. While seeing treatments for these conditions, many of which were entirely unmet before, is fantastic, the move into diseases of greater prevalence has the potential to impact the lives of many more patients.
More news and updates
.png)
.png)